Volume 33, Issue 2 (6-2024)
JGUMS 2024, 33(2): 176-187 |
Back to browse issues page
Research code: 99-3-56-19247
Ethics code: IR.IUMS.REC.1399.1374
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rastegari A, Mohammadi Z, Faghihi H, Rezaei S. Preparation and Physicochemical Evaluation of Eye Drop Formulation Based on Hyaluronic Acid and Vitamin B12. JGUMS 2024; 33 (2) :176-187
URL: http://journal.gums.ac.ir/article-1-2631-en.html
URL: http://journal.gums.ac.ir/article-1-2631-en.html
1- Department of Pharmaceutics and Pharmaceutical Nanotechnology, School of Pharmacy, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 2974 kb]
(391 Downloads)
| Abstract (HTML) (2047 Views)
Full-Text: (1202 Views)
Introduction
Dry eye is one of the age-related diseases, which is considered one of the most common eye diseases with more than 7 million affected people (9% of the population) in Iran. One of the most important known treatments for eye diseases is the use of eye drops, which in most cases can improve the patient’s condition. Among the effective drops, we can indicate Artelac Rebalance and Vitadrop eye drops, which contain vitamin B12 and hyaluronic acid. Vitamin B12 is an essential factor in maintaining eye health and has antioxidant properties that protect the surface of the eye from damage caused by harmful factors such as reactive oxygen species (ROS). Recently, injectable and topical forms of vitamin B12 are used for eye pain and dry eye treatment [4].
One of the most important factors determining clinical effectiveness and increasing patient acceptance is the drug’s efficacy duration. The short shelf life of eye drops can increase the need to use drops and reduce patient satisfaction [8, 9, 10, 11]. The high prevalence of eye diseases and the significant need for specific medicines and considering the economic conditions of the country make it necessary to prepare new eye treatment products with optimal effectiveness inside the country, which, while meeting the medical needs of the society, can help the economic development of the country. Due to the positive effects of vitamin B12 and hyaluronic acid, preparation and formulation of an eye drop with the presence of these components is necessary for the treatment of dry eye disease in Iranian people, which can provide a platform for competition even with international companies.
Methods
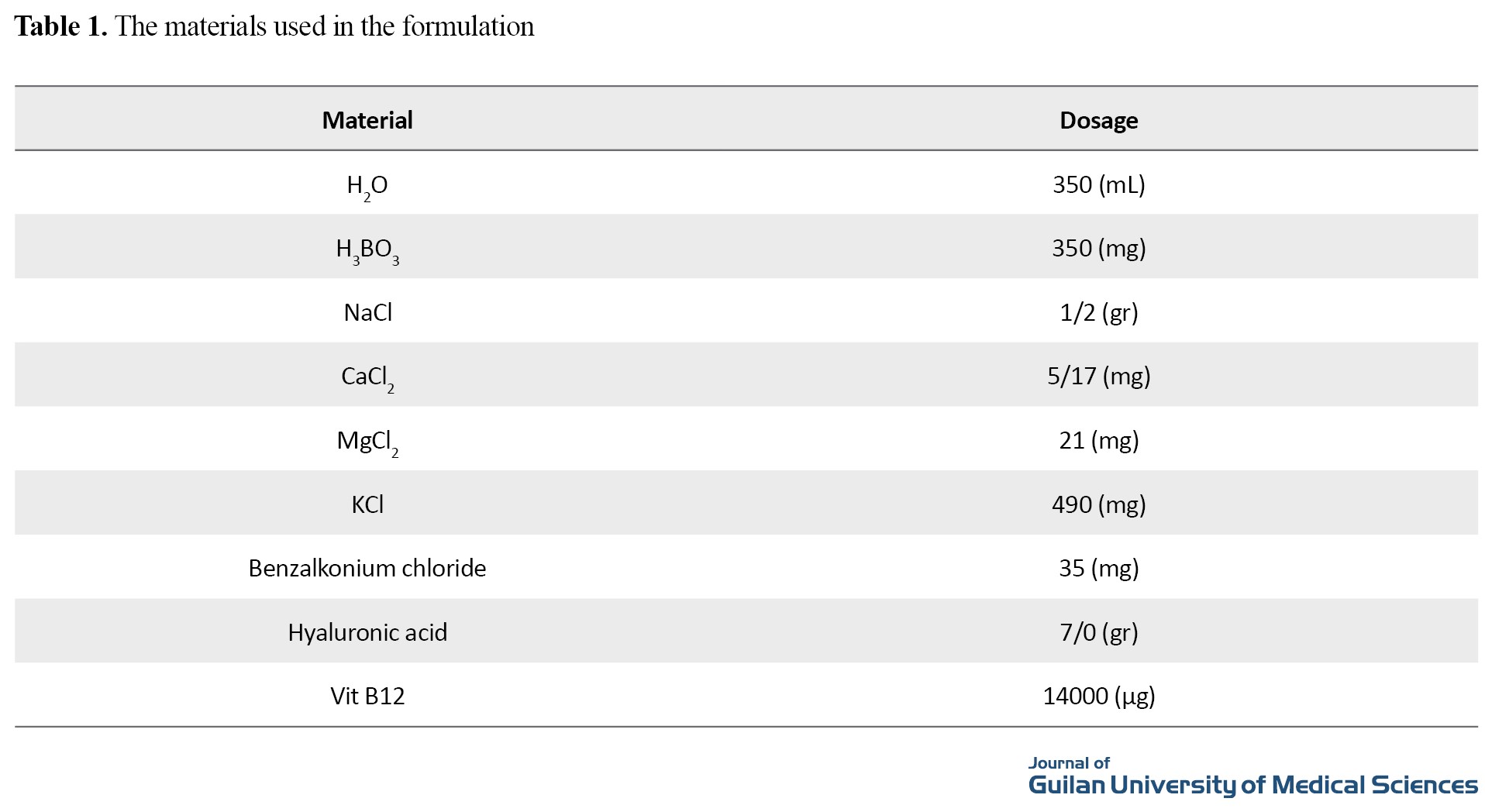
Vitamin B12 was obtained from Raha Pharmaceutical Company (Iran). Hyaluronic acid, boric acid and benzalkonium chloride were obtained from Sigma Aldrich Company (USA). The rest materials were obtained from Merck Company (Germany). To prepare eye drops containing 40 µg/ml vitamin B12 and 0.2% hyaluronic acid, the materials shown in Table 1 were mixed.
UV spectroscopy was used to check the changes in the concentration of vitamin B12 in the prepared formulation. The efficacy of the protective agent (benzalkonium chloride) was investigated according to the USP 43 pharmacopoeia and using the services of the Food and Drug Laboratory (Tavan Institute, Iran) after 6 months of stability testing. To check the stability of the prepared formulation, the eye drops were kept at a temperature of 40 and a humidity of 75% for 6 months. During this period, properties such as color, uniformity, the presence of particles in the formulation, the amount of B12 based on UV absorption, and the amount of osmolarity and viscosity were examined every month, and at the end of the study, the effectiveness of the protective agent was tested.
Results
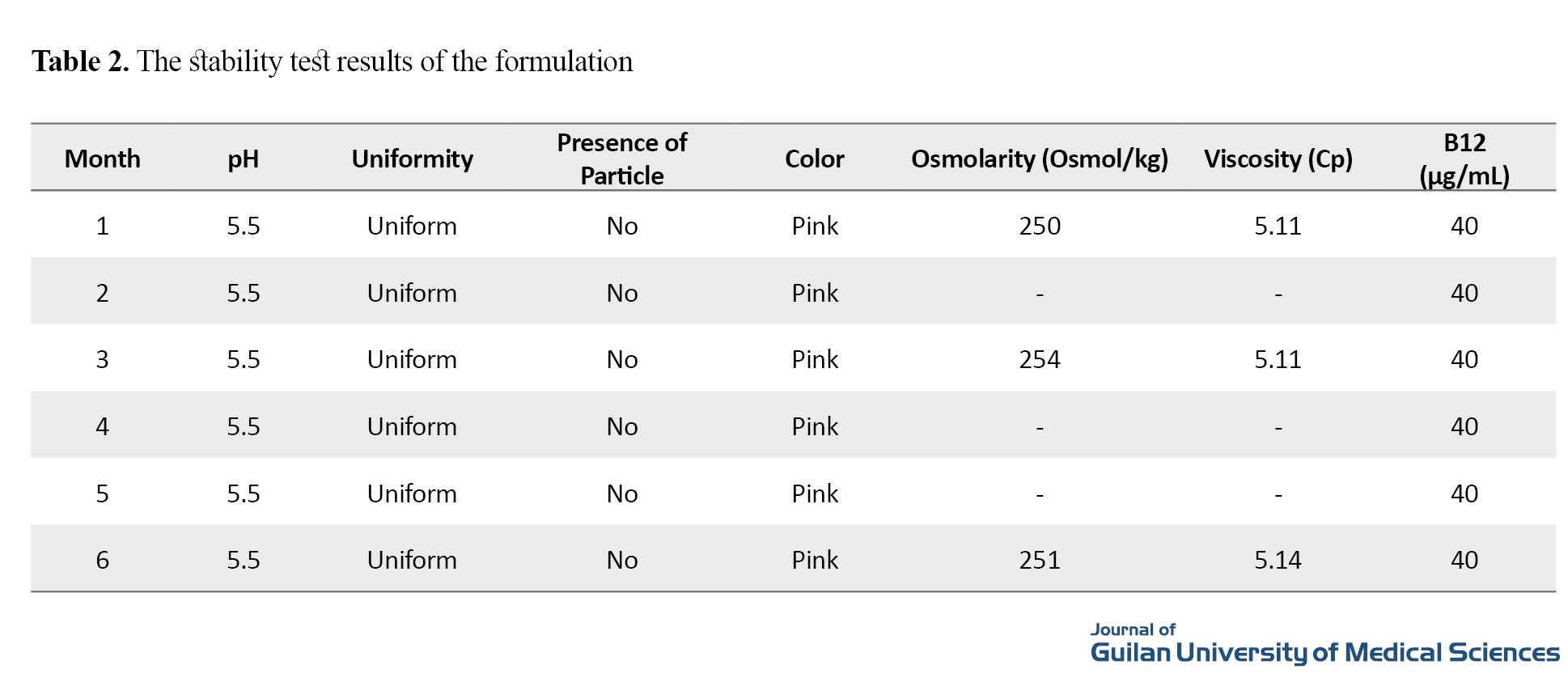
According to the results vitamin B12 had the maximum absorption at the wavelength of 361 nm. The standard curve for B12 is plotted. In the stability test, according to the obtained equation, the amount of vitamin B12 was evaluated at different times. The formulations prepared at different times were evaluated and the results of the accelerated stability test according to World Health Organization (WHO) guidelines after 6 months are presented in Table 2.
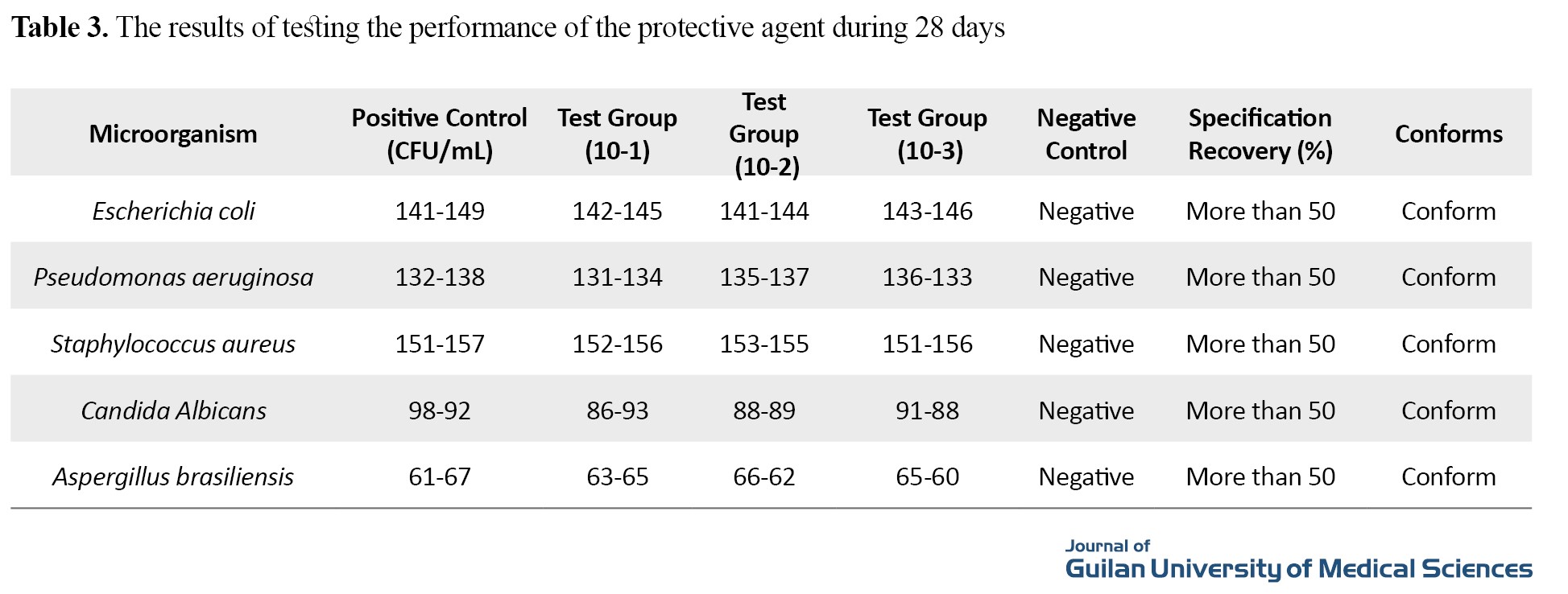
According to the results in Table 3, the selected protective agent (benzalkonium chloride) in the prepared concentration can be a suitable option to protect the ophthalmic formulation and the results are in accordance with the USP pharmacopoeia.
Conclusion
The results obtained from the formulation prepared in this study after the accelerated stability test according to the WHO guidelines showed that the eye drops containing vitamin B12 have good stability after 6 months and the amount of the effective substance has not changed. On the other hand, in this study, the minimum concentration of the protective agent benzalkonium chloride was used, which was able to maintain its effectiveness after 6 months of stability study. In this study, a 0.2% hyaluronic acid was used in the formulation, which has been shown in studies to effectively remove eye inflammation and to be effective in dry eye disease, with no any complications.
According to the obtained results, it seems that the formulation has the necessary efficiency as an artificial eye drop and can effectively deliver vitamin B12 to the eyes. This formulation with the minimum concentration of benzalkonium chloride can be a suitable option for the treatment of dry eyes with osmolarity, viscosity and pH similar to commercial and acceptable formulations in the pharmaceutical industry.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran. (Code: IR.IUMS.REC.1399.1374).
Funding
The present study was financially supported by the Vice-President of Research and Technology, Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and study design: Ali Rastegari, Zohreh Mohammadi, and Homa Faghihi; Data acquisition, analysis and curation: Ali Rastegari, Zohreh Mohammadi, and Sanaz Rezaei; Writing: Ali Rastegari, and Zohreh Mohammadi.
Conflicts of interest
The authors declared no conflict of interest.
References
Dry eye is one of the age-related diseases, which is considered one of the most common eye diseases with more than 7 million affected people (9% of the population) in Iran. One of the most important known treatments for eye diseases is the use of eye drops, which in most cases can improve the patient’s condition. Among the effective drops, we can indicate Artelac Rebalance and Vitadrop eye drops, which contain vitamin B12 and hyaluronic acid. Vitamin B12 is an essential factor in maintaining eye health and has antioxidant properties that protect the surface of the eye from damage caused by harmful factors such as reactive oxygen species (ROS). Recently, injectable and topical forms of vitamin B12 are used for eye pain and dry eye treatment [4].
One of the most important factors determining clinical effectiveness and increasing patient acceptance is the drug’s efficacy duration. The short shelf life of eye drops can increase the need to use drops and reduce patient satisfaction [8, 9, 10, 11]. The high prevalence of eye diseases and the significant need for specific medicines and considering the economic conditions of the country make it necessary to prepare new eye treatment products with optimal effectiveness inside the country, which, while meeting the medical needs of the society, can help the economic development of the country. Due to the positive effects of vitamin B12 and hyaluronic acid, preparation and formulation of an eye drop with the presence of these components is necessary for the treatment of dry eye disease in Iranian people, which can provide a platform for competition even with international companies.
Methods
Vitamin B12 was obtained from Raha Pharmaceutical Company (Iran). Hyaluronic acid, boric acid and benzalkonium chloride were obtained from Sigma Aldrich Company (USA). The rest materials were obtained from Merck Company (Germany). To prepare eye drops containing 40 µg/ml vitamin B12 and 0.2% hyaluronic acid, the materials shown in Table 1 were mixed.

UV spectroscopy was used to check the changes in the concentration of vitamin B12 in the prepared formulation. The efficacy of the protective agent (benzalkonium chloride) was investigated according to the USP 43 pharmacopoeia and using the services of the Food and Drug Laboratory (Tavan Institute, Iran) after 6 months of stability testing. To check the stability of the prepared formulation, the eye drops were kept at a temperature of 40 and a humidity of 75% for 6 months. During this period, properties such as color, uniformity, the presence of particles in the formulation, the amount of B12 based on UV absorption, and the amount of osmolarity and viscosity were examined every month, and at the end of the study, the effectiveness of the protective agent was tested.
Results
According to the results vitamin B12 had the maximum absorption at the wavelength of 361 nm. The standard curve for B12 is plotted. In the stability test, according to the obtained equation, the amount of vitamin B12 was evaluated at different times. The formulations prepared at different times were evaluated and the results of the accelerated stability test according to World Health Organization (WHO) guidelines after 6 months are presented in Table 2.

According to the results in Table 3, the selected protective agent (benzalkonium chloride) in the prepared concentration can be a suitable option to protect the ophthalmic formulation and the results are in accordance with the USP pharmacopoeia.

Conclusion
The results obtained from the formulation prepared in this study after the accelerated stability test according to the WHO guidelines showed that the eye drops containing vitamin B12 have good stability after 6 months and the amount of the effective substance has not changed. On the other hand, in this study, the minimum concentration of the protective agent benzalkonium chloride was used, which was able to maintain its effectiveness after 6 months of stability study. In this study, a 0.2% hyaluronic acid was used in the formulation, which has been shown in studies to effectively remove eye inflammation and to be effective in dry eye disease, with no any complications.
According to the obtained results, it seems that the formulation has the necessary efficiency as an artificial eye drop and can effectively deliver vitamin B12 to the eyes. This formulation with the minimum concentration of benzalkonium chloride can be a suitable option for the treatment of dry eyes with osmolarity, viscosity and pH similar to commercial and acceptable formulations in the pharmaceutical industry.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran. (Code: IR.IUMS.REC.1399.1374).
Funding
The present study was financially supported by the Vice-President of Research and Technology, Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and study design: Ali Rastegari, Zohreh Mohammadi, and Homa Faghihi; Data acquisition, analysis and curation: Ali Rastegari, Zohreh Mohammadi, and Sanaz Rezaei; Writing: Ali Rastegari, and Zohreh Mohammadi.
Conflicts of interest
The authors declared no conflict of interest.
References
- Doganay S, Guzel D, Ozdemir I, Yuvaci I. Evaluation of tear fluid and ocular dominance in patients with refractive error. International Journal of Health Services Research and Policy. 2018; 3(2):53-60. [Link]
- Holly FJ, Lemp MA. Tear physiology and dry eyes. Survey of Ophthalmology. 1977; 22(2):69-87. [DOI:10.1016/0039-6257(77)90087-X] [PMID]
- Tiffany J. Individual variations in human meibomian lipid composition. Experimental Eye Research. 1978; 27(3):289-300. [DOI:10.1016/0014-4835(78)90164-1] [PMID]
- Gurny R, Ryser JE, Tabatabay C, Martenet M, Edman P, Camber O. Precorneal residence time in humans of sodium hyaluronate as measured by gamma scintigraphy. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1990; 228(6):510-2. [DOI:10.1007/BF00918481] [PMID]
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. The Ocular Surface. 2017; 15(3):276-83. [DOI:10.1016/j.jtos.2017.05.008] [PMID]
- Partenhauser A, Bernkop-Schnürch A. Mucoadhesive polymers in the treatment of dry X syndrome. Drug Discovery Today. 2016; 21(7):1051-62. [DOI:10.1016/j.drudis.2016.02.013] [PMID]
- Snibson GR, Greaves JL, Soper ND, Prydal JI, Wilson CG, Bron AJ. Precorneal residence times of sodium hyaluronate solutions studied by quantitative gamma scintigraphy. Eye. 1990; 4(4):594-602. [DOI:10.1038/eye.1990.83] [PMID]
- Messmer EM. [Osmoprotection as a new therapeutic principle (German)]. Der Ophthalmologe. 2007; 104(11):987-90.[DOI:10.1007/s00347-007-1649-z] [PMID]
- Foulks GN. Pharmacological management of dry eye in the elderly patient. Drugs & Aging. 2008; 25(2):105-18.[DOI:10.2165/00002512-200825020-00003] [PMID]
- Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. The Ocular Surface. 2017; 15(3):575-628. [DOI:10.1016/j.jtos.2017.05.006] [PMID]
- Hasegawa T, Amako H, Yamamoto T, Tazawa M, Sakamoto Y. Corneal-protective effects of an artificial tear containing sodium hyaluronate and castor oil on a porcine short-term dry eye model. Journal of Veterinary Medical Science. 2014; 76(9):1219-24. [DOI:10.1292/jvms.14-0143] [PMID]
- Maïssa C, Guillon M, Simmons P, Vehige J. Effect of castor oil emulsion eyedrops on tear film composition and stability. Contact Lens and Anterior Eye. 2010; 33(2):76-82. [DOI:10.1016/j.clae.2009.10.005] [PMID]
- Johnson ME, Murphy PJ, Boulton M. Carbomer and sodium hyaluronate eyedrops for moderate dry eye treatment. Optometry and Vision Science. 2008; 85(8):750-7. [DOI:10.1097/OPX.0b013e318182476c] [PMID]
- Vogel R, Crockett RS, Oden N, Laliberte TW, Molina L; Sodium Hyaluronate Ophthalmic Solution Study Group. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). American Journal of Ophthalmology. 2010; 149(4):594-601. [DOI:10.1016/j.ajo.2009.09.023] [PMID]
- Pinto-Fraga J, López-de la Rosa A, Blázquez Arauzo F, Urbano Rodríguez R, González-García MJ. Efficacy and safety of 0.2% hyaluronic acid in the management of dry eye disease. Eye & Contact Lens. 2017; 43(1):57-63. [DOI:10.1097/ICL.0000000000000236] [PMID]
- United States Pharmacopeia. USP 39-NF34. Maryland: The United States Pharmacopeial; 2016.
- Kopp-Kubel S, Zahn M. The WHO stability guideline. In: Mazzo DJ, editor. International stability testing. Boca Raton: CRC Press; 2020. [DOI:10.1201/9781003076087-18]
- No Author. Omk2 eye drops [Internet]. 2024 [Updated 2024 June 29]. Available from: [Link]
- Mohamad SA, Alaaeldin E, Abdallah RM, Mansour HF. A new approach for dry eye management by mucoadhesive in situ gel of Vitamin B12: Formulation, In vitro and In vivo Assessment. AAPS PharmSciTech. 2021; 22(3):87. [DOI:10.1208/s12249-021-01957-4] [PMID]
- De-Hita-Cantalejo C, Sánchez-González MC, Silva-Viguera C, García-Romera MC, Feria-Mantero R, Sánchez-González JM. Efficacy of hyaluronic acid 0.3%, cyanocobalamin, electrolytes, and P-Plus in menopause patients with moderate dry eye disease. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2022; 260(2):529-35. [DOI:10.1007/s00417-021-05415-6] [PMID]
Review Paper: Research |
Subject:
Special
Received: 2023/08/16 | Accepted: 2024/02/12 | Published: 2024/07/1
Received: 2023/08/16 | Accepted: 2024/02/12 | Published: 2024/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









