Volume 33, Issue 2 (6-2024)
JGUMS 2024, 33(2): 134-159 |
Back to browse issues page
Research code: برگرفته از طرح نمیباشد.
Ethics code: این مقاله از نوع مروری است که هیچگونه آزمایشی بر روی ا
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rostamani H, Fakhraei O, Toosizadeh Khorasani F, Kelidari N. A Review of 3D Bioprinting Technologies in the Reconstruction of Human Nasal Cartilaginous Tissue. JGUMS 2024; 33 (2) :134-159
URL: http://journal.gums.ac.ir/article-1-2574-en.html
URL: http://journal.gums.ac.ir/article-1-2574-en.html
1- Department of Biomedical Engineering, Faculty of Engineering, Mashhad Branch, Islamic Azad University, Mashhad, Iran.
Full-Text [PDF 10714 kb]
(767 Downloads)
| Abstract (HTML) (2416 Views)
References
Full-Text: (1624 Views)
Introduction
Nasal cartilage is a specific connective tissue of hyaline type, without nerves, vessels and lymph, and has a very low number of cells. This tissue consists of approximately 1% chondrocytes and 99% extracellular matrix (ECM), and its chondrocytes originate from mesenchymal stem cells [1]. Cartilage ECM is mainly composed of water, macromolecules and proteoglycans, and is the product and the host of chondrocytes at the same time [3]. These features cause special mechanical properties and low self-healing capacity. Currently, the common and gold standard treatment is reconstruction using local flap or autologous cartilage transplantation (ACI) [1, 4]. The ACI surgery for major nasal reconstruction is a complex, time-consuming and skill-based procedure that involves harvesting rib cartilage, cutting and manually suturing them into the nose-shaped framework. The duration of the surgery can be more than 8 hours, during which the patient is under general anesthesia [4]. Due to the lack of septal cartilage, a modified autologous costal cartilage is usually used for grafting [2]. The purpose of cartilage tissue repair is to restore the key properties of native hyaline cartilage from the histological and biomechanical point of view.
In recent years, medical engineering applications for nasal cartilage have been rapidly developed [6, 7]. Bioprinting is an interdisciplinary science between medical sciences, biology, mechanical engineering and material science, and it is also known as a new approach in tissue engineering [12, 13]. As one of the branches of 3D printing, bioprinting technology is based on the precise placement of living cells and biomaterials to create replacement tissues based on the specific geometric and functional requirements of each tissue, with similar functions and properties. Bioprinting, in addition to avoiding complications of the donor area and personalization, has created unique opportunities for cartilage repair and reconstruction [4, 14]. This technology enables the printing of constructs with small and fine details up to dimensions with several hundreds of nanometers [15]. Today, it is also possible to simultaneously deposit two or more biological substances and produce complex and personalized constructs based on the patient’s medical images [21].
Bioprinting has been able to overcome many limitations of tissue engineering, such as accurate cell distribution and tissue biological function; however, there are challenges in nasal cartilage reconstruction with bioprinting. One of the most important challenges is the unavailability of appropriate bioinks [18, 22]. This study aimed to investigate the ability of different three-dimensional bioprinting methods to repair defects in the nose area. In this regard, the studies with on nasal cartilage bioprinting, related technologies and its process were reviewed.
Methods
This is a review study. The search for related articles was done in ScienceDirect, Springer, Wiley, Cambridge, DeGruyter and Google Scholar databases using the keywords tissue engineering, nasal cartilage, 3D bioprinting, and bioink materials and with a time limit of 4 years. Out of 300 articles, 159 were finally included for the review.
Results
The review of studied showed that, in order to obtain the desired results in vitro or in vivo, choosing the appropriate bioprinting approach and biological ink has a major role. The most important studies with in-vitro tests are listed in Table 1.
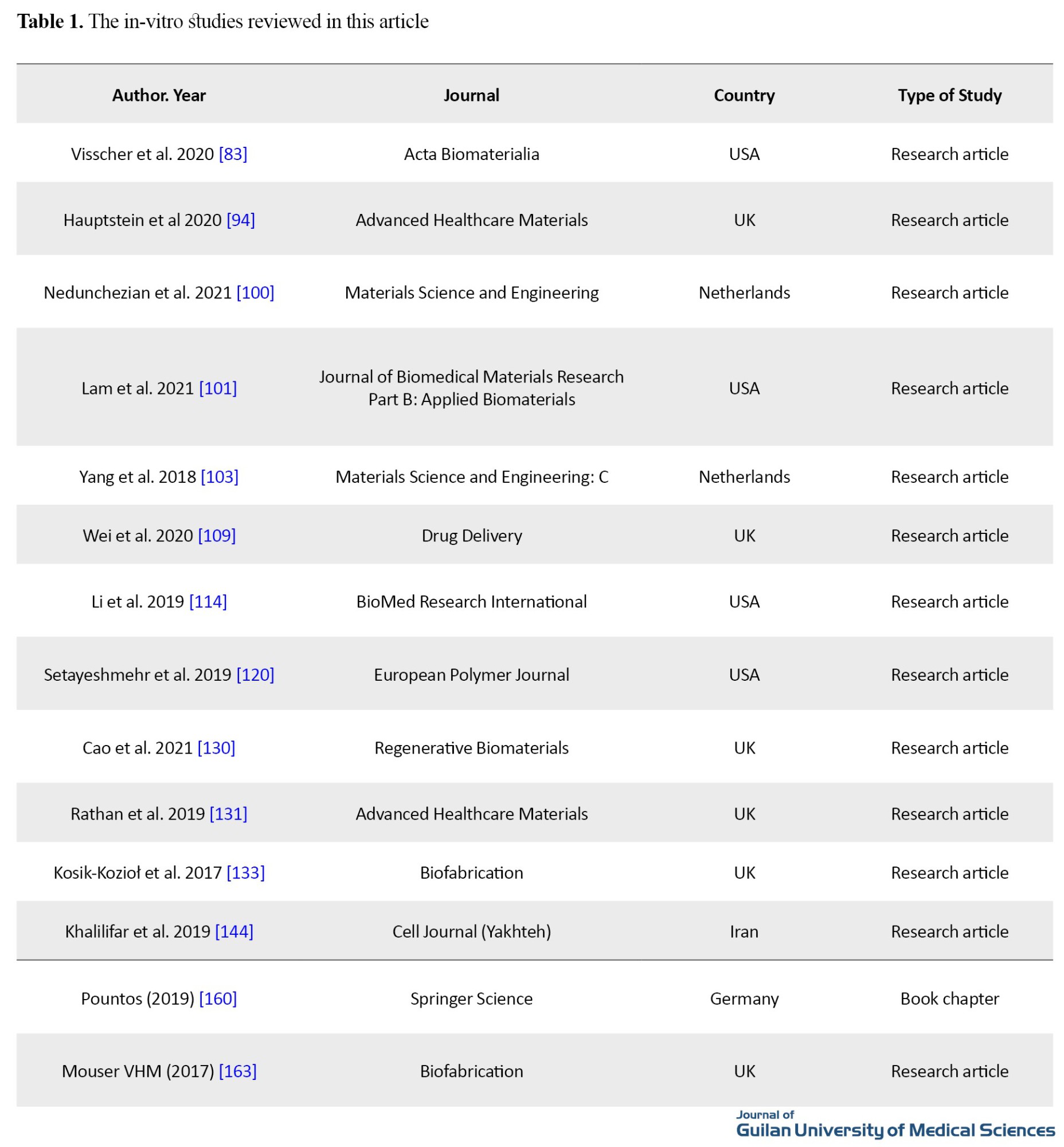
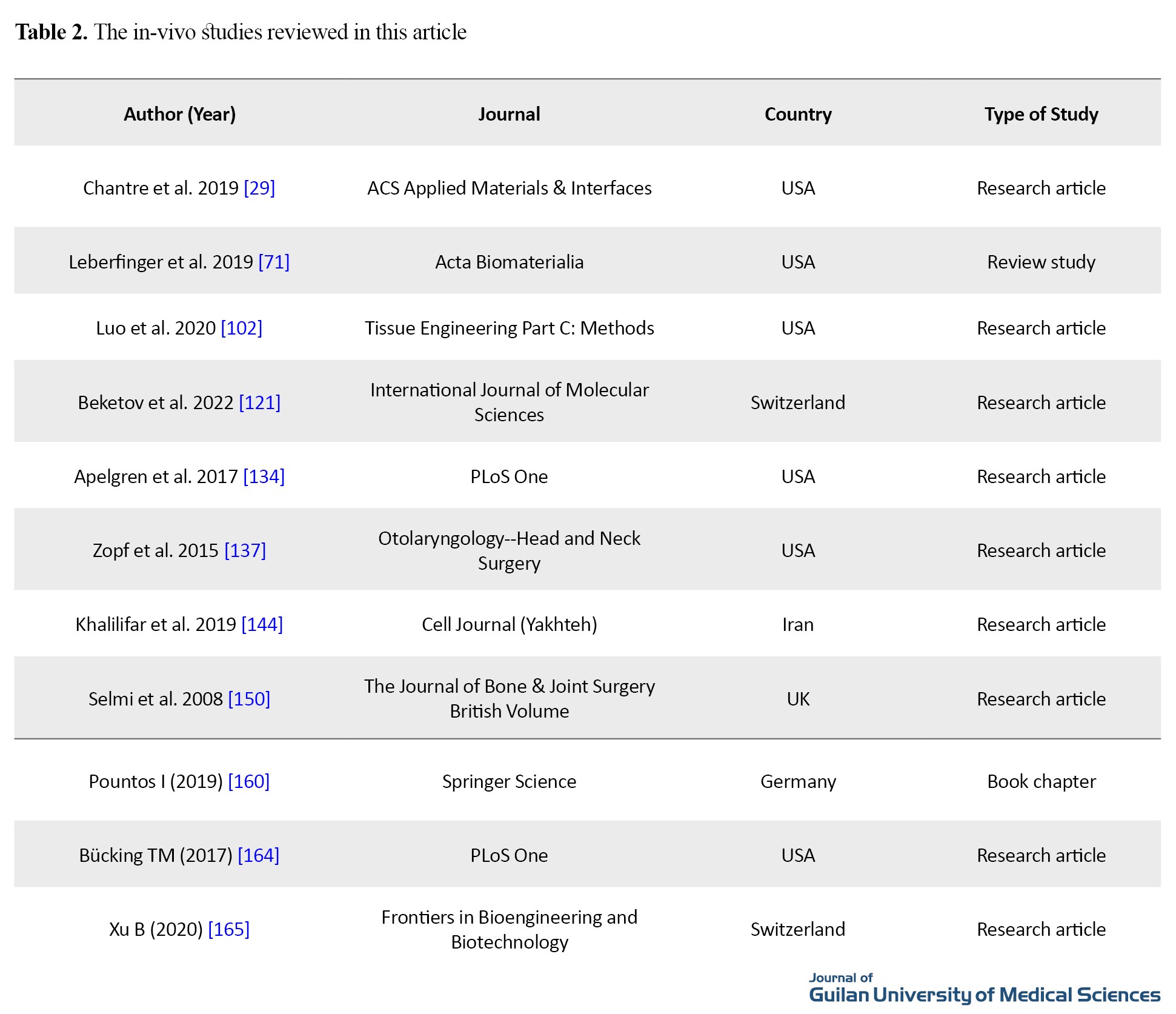
Table 2 shows the studies with in-vivo tests.
The presented findings related to 3D bioprinting technologies included the processing method, extrusion method, inkjet method, laser method, application in the field of nasal cartilage, characteristics of nasal cartilage, nasal cartilage implantation, 3D modeling, bio ink, bio materials, cell sources, bioactives, in-vitro evaluations, and in-vivo evaluations.
Conclusion
Until recently, cartilage tissue regeneration methods were limited to the use of autologous or allogeneic cartilages or the use of alloplastic grafts, each of which had inevitable disadvantages. For this reason, bioprinting is used to solve these problems in regenerative medicine. According to the specific requirements of the bioinks of the target tissues, researchers have used different technologies for tissue and organ printing, each of which has advantages and limitations. Based on the in-vivo and in-vitro studies that have been carried out on the printed nasal cartilage tissues, bioprinting technology has been able to imitate the cartilage host tissue to a very acceptable extent in terms of morphological, biochemical and mechanical aspects. Although the use of this technology at the clinical level still faces limitations, the prospect of this technology is promising since it can address nasal defects with low cost, unique accuracy and personalization.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design, data acquisition, analysis and curation: Fatemeh Toosizadeh Khorasani and Hosein Rostamani; Statistic analysis and initial draft preparation: Hosein Rostamani; Review and editing: Omid Fakhraei and Narges Kelidari; Supervision: Omid Fakhraei.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Department of Medical Engineering, Islamic Azad University, Mashhad Branch, Iran, Mashhad for accompanying in this research.
Nasal cartilage is a specific connective tissue of hyaline type, without nerves, vessels and lymph, and has a very low number of cells. This tissue consists of approximately 1% chondrocytes and 99% extracellular matrix (ECM), and its chondrocytes originate from mesenchymal stem cells [1]. Cartilage ECM is mainly composed of water, macromolecules and proteoglycans, and is the product and the host of chondrocytes at the same time [3]. These features cause special mechanical properties and low self-healing capacity. Currently, the common and gold standard treatment is reconstruction using local flap or autologous cartilage transplantation (ACI) [1, 4]. The ACI surgery for major nasal reconstruction is a complex, time-consuming and skill-based procedure that involves harvesting rib cartilage, cutting and manually suturing them into the nose-shaped framework. The duration of the surgery can be more than 8 hours, during which the patient is under general anesthesia [4]. Due to the lack of septal cartilage, a modified autologous costal cartilage is usually used for grafting [2]. The purpose of cartilage tissue repair is to restore the key properties of native hyaline cartilage from the histological and biomechanical point of view.
In recent years, medical engineering applications for nasal cartilage have been rapidly developed [6, 7]. Bioprinting is an interdisciplinary science between medical sciences, biology, mechanical engineering and material science, and it is also known as a new approach in tissue engineering [12, 13]. As one of the branches of 3D printing, bioprinting technology is based on the precise placement of living cells and biomaterials to create replacement tissues based on the specific geometric and functional requirements of each tissue, with similar functions and properties. Bioprinting, in addition to avoiding complications of the donor area and personalization, has created unique opportunities for cartilage repair and reconstruction [4, 14]. This technology enables the printing of constructs with small and fine details up to dimensions with several hundreds of nanometers [15]. Today, it is also possible to simultaneously deposit two or more biological substances and produce complex and personalized constructs based on the patient’s medical images [21].
Bioprinting has been able to overcome many limitations of tissue engineering, such as accurate cell distribution and tissue biological function; however, there are challenges in nasal cartilage reconstruction with bioprinting. One of the most important challenges is the unavailability of appropriate bioinks [18, 22]. This study aimed to investigate the ability of different three-dimensional bioprinting methods to repair defects in the nose area. In this regard, the studies with on nasal cartilage bioprinting, related technologies and its process were reviewed.
Methods
This is a review study. The search for related articles was done in ScienceDirect, Springer, Wiley, Cambridge, DeGruyter and Google Scholar databases using the keywords tissue engineering, nasal cartilage, 3D bioprinting, and bioink materials and with a time limit of 4 years. Out of 300 articles, 159 were finally included for the review.
Results
The review of studied showed that, in order to obtain the desired results in vitro or in vivo, choosing the appropriate bioprinting approach and biological ink has a major role. The most important studies with in-vitro tests are listed in Table 1.

Table 2 shows the studies with in-vivo tests.

The presented findings related to 3D bioprinting technologies included the processing method, extrusion method, inkjet method, laser method, application in the field of nasal cartilage, characteristics of nasal cartilage, nasal cartilage implantation, 3D modeling, bio ink, bio materials, cell sources, bioactives, in-vitro evaluations, and in-vivo evaluations.
Conclusion
Until recently, cartilage tissue regeneration methods were limited to the use of autologous or allogeneic cartilages or the use of alloplastic grafts, each of which had inevitable disadvantages. For this reason, bioprinting is used to solve these problems in regenerative medicine. According to the specific requirements of the bioinks of the target tissues, researchers have used different technologies for tissue and organ printing, each of which has advantages and limitations. Based on the in-vivo and in-vitro studies that have been carried out on the printed nasal cartilage tissues, bioprinting technology has been able to imitate the cartilage host tissue to a very acceptable extent in terms of morphological, biochemical and mechanical aspects. Although the use of this technology at the clinical level still faces limitations, the prospect of this technology is promising since it can address nasal defects with low cost, unique accuracy and personalization.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, study design, data acquisition, analysis and curation: Fatemeh Toosizadeh Khorasani and Hosein Rostamani; Statistic analysis and initial draft preparation: Hosein Rostamani; Review and editing: Omid Fakhraei and Narges Kelidari; Supervision: Omid Fakhraei.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Department of Medical Engineering, Islamic Azad University, Mashhad Branch, Iran, Mashhad for accompanying in this research.
References
- Chiesa-Estomba CM, Aiastui A, González-Fernández I, Hernáez-Moya R, Rodiño C, Delgado A, et al. Three-dimensional bioprinting scaffolding for nasal cartilage defects: A systematic review. Tissue Engineering and Regenerative Medicine. 2021; 18(3):343-53. [DOI:10.1007/s13770-021-00331-6] [PMID]
- Lan X, Liang Y, Vyhlidal M, Erkut EJ, Kunze M, Mulet-Sierra A, et al. In vitro maturation and in vivo stability of bioprinted human nasal cartilage. Journal of Tissue Engineering. 2022; 13:20417314221086368. [DOI:10.1177/20417314221086368] [PMID]
- Agarwal T, Chiesa I, Presutti D, Irawan V, Vajanthri KY, Costantini M, et al. Recent advances in bioprinting technologies for engineering different cartilage-based tissues. Materials Science and Engineering: C. 2021; 123:112005. [DOI:10.1016/j.msec.2021.112005] [PMID]
- Ruiz-Cantu L, Gleadall A, Faris C, Segal J, Shakesheff K, Yang J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Materials Science & Engineering. C, Materials for Biological Applications. 2020; 109:110578. [DOI:10.1016/j.msec.2019.110578] [PMID]
- Wang H, Wang Z, Liu H, Liu J, Li R, Zhu X, et al. Three-dimensional printing strategies for irregularly shaped cartilage tissue engineering: Current state and challenges. Frontiers in Bioengineering and Biotechnology. 2022; 9:777039. [DOI:10.3389/fbioe.2021.777039] [PMID]
- Shi B, Huang H. Computational technology for nasal cartilage-related clinical research and application. International Journal of Oral Science. 2020; 12(1):21. [DOI:10.1038/s41368-020-00089-y] [PMID]
- Alkaya D, Gurcan C, Kilic P, Yilmazer A, Gurman G. Where is human-based cellular pharmaceutical R&D taking us in cartilage regeneration? 3 Biotech. 2020; 10(4):161. [DOI:10.1007/s13205-020-2134-5] [PMID]
- Eshkalak SK, Ghomi ER, Dai Y, Choudhury D, Ramakrishna S. The role of three-dimensional printing in healthcare and medicine. Materials & Design. 2020; 194:108940. [DOI:10.1016/j.matdes.2020.108940]
- Bigham A, Foroughi F, Rezvani Ghomi E, Rafienia M, Neisiany RE, Ramakrishna S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-Design and Manufacturing. 2020; 3:281-306. [DOI:10.1007/s42242-020-00094-4]
- Osborn LS. 3D printing and intellectual property. Cambridge: Cambridge University Press; 2019. [DOI:10.1017/9781316584507]
- Jian H, Wang M, Wang S, Wang A, Bai S. 3D bioprinting for cell culture and tissue fabrication. Bio-Design and Manufacturing. 2018; 1:45-61. [DOI:10.1007/s42242-018-0006-1]
- Xie Z, Gao M, Lobo AO, Webster TJ. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers. 2020; 12(8):1717. [DOI:10.3390/polym12081717]
- Ghasemian Fard M, Sharifianjazi F, Kazemi SS, Rostamani H, Bathaei MS. Laser-based additive manufacturing of magnesium alloys for bone tissue engineering applications: From chemistry to clinic. Journal of Manufacturing and Materials Processing. 2022; 6(6):158. [DOI:10.3390/jmmp6060158]
- Stampoultzis T, Karami P, Pioletti DP. Thoughts on cartilage tissue engineering: A 21st century perspective. Current Research in Translational Medicine. 2021; 69(3):103299. [DOI:10.1016/j.retram.2021.103299] [PMID]
- You S, Li J, Zhu W, Yu C, Mei D, Chen S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. Journal of Materials Chemistry B. 2018; 6(15):2187-97. [DOI:10.1039/C8TB00301G] [PMID]
- Thayer P, Martinez H, Gatenholm E. History and Trends of 3D Bioprinting. Methods in Molecular Biology. 2020; 2140:3-18.[DOI:10.1007/978-1-0716-0520-2_1] [PMID]
- Ning L, Yang B, Mohabatpour F, Betancourt N, Sarker MD, Papagerakis P, et al. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication. 2020; 12(2):025011. [DOI:10.1088/1758-5090/ab5f53] [PMID]
- Fu Z, Naghieh S, Xu C, Wang C, Sun W, Chen X. Printability in extrusion bioprinting. Biofabrication. 2021; 13(3):033001. [DOI:10.1088/1758-5090/abe7ab] [PMID]
- Schwab A, Levato R, D'Este M, Piluso S, Eglin D, Malda J. Printability and shape fidelity of bioinks in 3D bioprinting. Chemical Reviews. 2020; 120(19):11028-55. [DOI:10.1021/acs.chemrev.0c00084] [PMID]
- Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Advanced Materials. 2018; 30(27):1800242. [DOI:10.1002/adma.201800242]
- Gupta N, Cruz MA, Nasser P, Rosenberg JD, Iatridis JC. Fibrin-genipin hydrogel for cartilage tissue engineering in nasal reconstruction. Annals of Otology, Rhinology & Laryngology. 2019; 128(7):640-6. [DOI:10.1177/0003489419836667] [PMID]
- Zhang S, Wang H. Current progress in 3D bioprinting of tissue analogs. SLAS Technology. 2019; 24(1):70-8. [DOI:10.1177/2472630318799971] [PMID]
- Kasper C, Egger D, Lavrentieva A. Basic concepts on 3d cell culture. Berlin: Springer; 2021. [DOI:10.1007/978-3-030-66749-8]
- Singh M, Jonnalagadda S. Advances in bioprinting using additive manufacturing. European Journal of Pharmaceutical Sciences. 2020; 143:105167. [DOI:10.1016/j.ejps.2019.105167] [PMID]
- Highley CB, Song KH, Daly AC, Burdick JA. Jammed microgel inks for 3D printing applications. Advanced Science. 2018; 6(1):1801076. [DOI:10.1002/advs.201801076] [PMID]
- Highley CB. 3D bioprinting technologies. In: Guvendiren M. 3D bioprinting in medicine: Technologies, bioinks, and applications. Berlin: Springer; 2019. [DOI:10.1007/978-3-030-23906-0_1]
- Xing F, Xiang Z, Rommens PM, Ritz U. 3D bioprinting for vascularized tissue-engineered bone fabrication. Materials. 2020; 13(10):2278. [DOI:10.3390/ma13102278] [PMID]
- Ramesh S, Harrysson OL, Rao PK, Tamayol A, Cormier DR, Zhang Y, et al. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting. 2021; 21:e00116. [DOI:10.1016/j.bprint.2020.e00116]
- Chantre CO, Gonzalez GM, Ahn S, Cera L, Campbell PH, Hoerstrup SP, et al. Porous biomimetic hyaluronic acid and extracellular matrix protein nanofiber scaffolds for accelerated cutaneous tissue repair. ACS Applied Materials & Interfaces. 2019; 11(49):45498-510. [DOI:10.1021/acsami.9b17322] [PMID]
- Choudhury D, Anand S, Naing MW. The arrival of commercial bioprinters - Towards 3D bioprinting revolution! International Journal of Bioprinting. 2018; 4(2):139. [DOI:10.18063/ijb.v4i2.139] [PMID]
- Maniruzzaman M. 3D and 4D printing in biomedical applications: Process engineering and additive manufacturing. New Jersey: John Wiley & Sons; 2019. [DOI:10.1002/9783527813704]
- Ravanbakhsh H, Bao G, Luo Z, Mongeau LG, Zhang YS. Composite inks for extrusion printing of biological and biomedical constructs. ACS Biomaterials Science & Engineering. 2021; 7(9):4009-26. [DOI:10.1021/acsbiomaterials.0c01158] [PMID]
- Huang J, Xiong J, Wang D, Zhang J, Yang L, Sun S, et al. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels. 2021; 7(3):144. [DOI:10.3390/gels7030144] [PMID]
- Pedroza-González SC, Rodriguez-Salvador M, Pérez-Benítez BE, Alvarez MM, Santiago GT. Bioinks for 3D bioprinting: A scientometric analysis of two decades of progress. International Journal of Bioprinting. 2021; 7(2):333. [DOI:10.18063/ijb.v7i2.337] [PMID]
- Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Advanced Healthcare Materials. 2017; 6(1):1601118. [DOI:10.1002/adhm.201601118] [PMID]
- Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, et al. Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication. 2018; 10(2):024102. [DOI:10.1088/1758-5090/aa9d44] [PMID]
- Unagolla JM, Jayasuriya AC. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Applied Materials Today. 2020; 18:100479. [DOI:10.1016/j.apmt.2019.100479] [PMID]
- Lee JM, Sing SL, Zhou M, Yeong WY. 3D bioprinting processes: A perspective on classification and terminology. International Journal of Bioprinting. 2018; 4(2):151. [DOI:10.18063/ijb.v4i2.151] [PMID]
- Jacob GT, Passamai VE, Katz S, Castro GR, Alvarez V. Hydrogels for extrusion-based bioprinting: General considerations. Bioprinting. 2022; 27:e00212. [DOI:10.1016/j.bprint.2022.e00212]
- Mao H, Yang L, Zhu H, Wu L, Ji P, Yang J, et al. Recent advances and challenges in materials for 3D bioprinting. Progress in Natural Science: Materials International. 2020; 30(5):618-34. [DOI:10.1016/j.pnsc.2020.09.015]
- Tetsuka H, Shin SR. Materials and technical innovations in 3D printing in biomedical applications. Journal of Materials Chemistry B. 2020; 8(15):2930-50. [DOI:10.1039/D0TB00034E] [PMID]
- Kang D, Hong G, An S, Jang I, Yun WS, Shim JH, et al. Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small. 2020; 16(13):1905505. [DOI:10.1002/smll.201905505]
- Betancourt N, Chen X. Review of extrusion-based multi-material bioprinting processes. Bioprinting. 2022; 25:e00189.[DOI:10.1016/j.bprint.2021.e00189]
- Jiang T, Munguia-Lopez JG, Flores-Torres S, Grant J, Vijayakumar S, Leon-Rodriguez AD, et al. Directing the self-assembly of tumour spheroids by bioprinting cellular heterogeneous models within alginate/gelatin hydrogels. Scientific Reports. 2017; 7(1):4575. [DOI:10.1038/s41598-017-04691-9] [PMID]
- Zhou LY, Gao Q, Fu JZ, Chen QY, Zhu JP, Sun Y, et al. Multimaterial 3D printing of highly stretchable silicone elastomers. ACS Applied Materials & Interfaces. 2019; 11(26):23573-83. [DOI:10.1021/acsami.9b04873] [PMID]
- Tan B, Gan S, Wang X, Liu W, Li X. Applications of 3D bioprinting in tissue engineering: Advantages, deficiencies, improvements, and future perspectives. Journal of Materials Chemistry B. 2021; 9(27):5385-413. [DOI:10.1039/D1TB00172H] [PMID]
- Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnology Journal. 2015; 10(10):1568-77. [DOI:10.1002/biot.201400635] [PMID]
- Zhang Z, Jin Y, Yin J, Xu C, Xiong R, Christensen K, et al. Evaluation of bioink printability for bioprinting applications. Applied Physics Reviews. 2018; 5(4):041304. [DOI:10.1063/1.5053979]
- Miri AK, Mirzaee I, Hassan S, Mesbah Oskui S, Nieto D, Khademhosseini A, et al. Effective bioprinting resolution in tissue model fabrication. Lab On A Chip. 2019; 19(11):2019-37. [DOI:10.1039/C8LC01037D] [PMID]
- Shen Y, Tang H, Huang X, Hang R, Zhang X, Wang Y, et al. DLP printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydrate Polymers. 2020; 235:115970. [DOI:10.1016/j.carbpol.2020.115970] [PMID]
- Lee JM, Ng WL, Yeong WY. Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing. Applied Physics Reviews. 2019; 6(1):011307. [DOI:10.1063/1.5053909]
- Du X. 3D bio-printing review. Paper presented at: IOP Conference Series: Materials Science and Engineering. 15–17 December 2017, Xiamen, China. [Link]
- Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z. 3D bioprinting: A novel avenue for manufacturing tissues and organs. Engineering. 2019; 5(4):777-94. [DOI:10.1016/j.eng.2019.03.009]
- Zhou D, Chen J, Liu B, Zhang X, Li X, Xu T. Bioinks for jet-based bioprinting. Bioprinting. 2019; 16:e00060. [DOI:10.1016/j.bprint.2019.e00060]
- Kumar K, Zindani D, Davim JP. Rapid prototyping, rapid tooling and reverse engineering: From biological models to 3d bioprinters: Walter de Gruyter GmbH & Co KG; 2020. [DOI:10.1515/9783110664904]
- Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioactive Materials. 2018; 3(2):144-56. [DOI:10.1016/j.bioactmat.2017.11.008] [PMID]
- Zhang B, Luo Y, Ma L, Gao L, Li Y, Xue Q, et al. 3D bioprinting: An emerging technology full of opportunities and challenges. Bio-Design and Manufacturing. 2018; 1:2-13. [DOI:10.1007/s42242-018-0004-3]
- Li H, Tan C, Li L. Review of 3D printable hydrogels and constructs. Materials & Design. 2018; 159:20-38. [DOI:10.1016/j.matdes.2018.08.023]
- Khoeini R, Nosrati H, Akbarzadeh A, Eftekhari A, Kavetskyy T, Khalilov R, et al. Natural and synthetic bioinks for 3D bioprinting. Advanced NanoBiomed Research. 2021; 1(8):2000097. [DOI:10.1002/anbr.202000097]
- Mondschein RJ, Kanitkar A, Williams CB, Verbridge SS, Long TE. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials. 2017; 140:170-88. [DOI:10.1016/j.biomaterials.2017.06.005] [PMID]
- Ong LJY, Islam A, DasGupta R, Iyer NG, Leo HL, Toh YC. A 3D printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication. 2017; 9(4):045005. [DOI:10.1088/1758-5090/aa8858] [PMID]
- Raman R, Bhaduri B, Mir M, Shkumatov A, Lee MK, Popescu G, et al. High-resolution projection microstereolithography for patterning of neovasculature. Advanced Healthcare Materials. 2016; 5(5):610-9. [DOI:10.1002/adhm.201500721] [PMID]
- Magalhães LSSM, Santos FEP, Elias CMV, Afewerki S, Sousa GF, Furtado ASA, et al. Printing 3D hydrogel structures employing low-cost stereolithography technology. Journal of Functional Biomaterials. 2020; 11(1):12. [DOI:10.3390/jfb11010012] [PMID]
- De S, Jose J, Pal A, Roy Choudhury S, Roy S. Exposure to low UV-B dose induces DNA double-strand breaks mediated onset of endoreduplication in Vigna radiata (L.) R. Wilczek seedlings. Plant and Cell Physiology. 2022; 63(4):463-83. [DOI:10.1093/pcp/pcac012] [PMID]
- Kumar H, Kim K. Stereolithography 3D Bioprinting. Methods in molecular biology (Clifton, N.J.). 2020; 2140:93-108.[DOI:10.1007/978-1-0716-0520-2_6] [PMID]
- Moura D, Pereira RF, Goncalves IC. Recent advances on bioprinting of hydrogels containing carbon materials. Materials Today Chemistry. 2022; 23:100617. [DOI:10.1016/j.mtchem.2021.100617]
- Xiang Y, Miller K, Guan J, Kiratitanaporn W, Tang M, Chen S. 3D bioprinting of complex tissues in vitro: State-of-the-art and future perspectives. Archives of Toxicology. 2022; 96(3):691-710. [DOI:10.1007/s00204-021-03212-y] [PMID]
- Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 2017; 124:106-15. [DOI:10.1016/j.biomaterials.2017.01.042] [PMID]
- Xia N, Jin D, Iacovacci V, Zhang L. 3D printing of functional polymers for miniature machines. Multifunctional Materials. 2022; 5(1):012001. [DOI:10.1088/2399-7532/ac4836]
- Debnath SK, Debnath M, Srivastava R, Omri A. Intervention of 3D printing in health care: Transformation for sustainable development. Expert Opinion on Drug Delivery. 2021; 18(11):1659-72. [DOI:10.1080/17425247.2021.1981287] [PMID]
- Leberfinger AN, Dinda S, Wu Y, Koduru SV, Ozbolat V, Ravnic DJ, et al. Bioprinting functional tissues. Acta Biomaterialia. 2019; 95:32-49. [DOI:10.1016/j.actbio.2019.01.009] [PMID]
- Han D, Yang C, Fang NX, Lee H. Rapid multi-material 3D printing with projection micro-stereolithography using dynamic fluidic control. Additive Manufacturing. 2019; 27:606-15. [DOI:10.1016/j.addma.2019.03.031]
- Kim YT, Castro K, Bhattacharjee N, Folch A. Digital manufacturing of selective porous barriers in microchannels using multi-material stereolithography. Micromachines. 2018; 9(3):125. [DOI:10.3390/mi9030125] [PMID]
- McGivern S, Boutouil H, Al-Kharusi G, Little S, Dunne NJ, Levingstone TJ. Translational application of 3D bioprinting for cartilage tissue engineering. Bioengineering. 2021; 8(10):144. [DOI:10.3390/bioengineering8100144] [PMID]
- Baddam P, Bayona-Rodriguez F, Campbell SM, El-Hakim H, Graf D. Properties of the nasal cartilage, from development to adulthood: A scoping review. Cartilage. 2022; 13(1):19476035221087696.[DOI:10.1177/19476035221087696] [PMID]
- Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nature Biomedical Engineering. 2020; 4(4):370-80. [DOI:10.1038/s41551-019-0471-7] [PMID]
- Agarwal S, Saha S, Balla VK, Pal A, Barui A, Bodhak S. Current developments in 3D bioprinting for tissue and organ regeneration-A review. Frontiers in Mechanical Engineering. 2020; 6:589171. [DOI:10.3389/fmech.2020.589171]
- Lavernia L, Brown WE, Wong BJ, Hu JC, Athanasiou KA. Toward tissue-engineering of nasal cartilages. Acta Biomaterialia. 2019; 88:42-56. [DOI:10.1016/j.actbio.2019.02.025] [PMID]
- Sun Y, Zhao Z, An Y. Application of digital technology in nasal reconstruction. Chinese Journal of Plastic and Reconstructive Surgery. 2021; 3(4):204-8. [DOI:10.1016/j.cjprs.2021.12.001]
- Wade DE. 3D Printing, Valuation, and Service Inputs: Looking to the future rather than the past to design rules of origin for advanced manufactured products. Brexit Institute Working Paper Series. 2024; No 02/2024. [Link]
- Wang X, Zhao L, Fuh JYH, Lee HP. Effect of porosity on mechanical properties of 3D printed polymers: Experiments and micromechanical modeling based on X-ray computed tomography analysis. Polymers. 2019; 11(7):1154. [DOI:10.3390/polym11071154] [PMID]
- Fay CD. Computer-Aided Design and Manufacturing (CAD/CAM) for Bioprinting. Methods in Molecular Biology. 2020; 2140:27-41. [DOI:10.1007/978-1-0716-0520-2_3] [PMID]
- Visscher DO, Lee H, van Zuijlen PPM, Helder MN, Atala A, Yoo JJ, et al. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomaterialia. 2021; 121:193-203. [DOI:10.1016/j.actbio.2020.11.029] [PMID]
- Chimene D, Lennox KK, Kaunas RR, Gaharwar AK. Advanced bioinks for 3D printing: A materials science perspective. Annals of Biomedical Engineering. 2016; 44(6):2090-102. [DOI:10.1007/s10439-016-1638-y] [PMID]
- Bian L. Functional hydrogel bioink, a key challenge of 3D cellular bioprinting. APL Bioengineering. 2020; 4(3):030401.[DOI:10.1063/5.0018548] [PMID]
- Sodupe-Ortega E, Sanz-Garcia A, Pernia-Espinoza A, Escobedo-Lucea C. Accurate calibration in multi-material 3D bioprinting for tissue engineering. Materials. 2018; 11(8):1402. [DOI:10.3390/ma11081402] [PMID]
- GhavamiNejad A, Ashammakhi N, Wu XY, Khademhosseini A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small. 2020; 16(35):2002931. [DOI:10.1002/smll.202002931]
- Ashammakhi N, Kaarela O. Three-dimensional bioprinting can help bone. Journal of Craniofacial Surgery. 2018; 29(1):9-11. [DOI:10.1097/SCS.0000000000004143] [PMID]
- Li Y, Liu Y, Jiang C, Li S, Liang G, Hu Q. A reactor-like spinneret used in 3D printing alginate hollow fiber: a numerical study of morphological evolution. Soft Matter. 2016; 12(8):2392-9. [DOI:10.1039/C5SM02733K] [PMID]
- Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends in Biotechnology. 2016; 34(5):394-407. [DOI:10.1016/j.tibtech.2016.01.002] [PMID]
- Wang Z, Yang Y, Gao Y, Xu Z, Yang S, Jin M. Establishing a novel 3D printing bioinks system with recombinant human collagen. International Journal of Biological Macromolecules. 2022; 211:400-9. [DOI:10.1016/j.ijbiomac.2022.05.088] [PMID]
- Oshida Y, Miyazaki T. Biomaterials and engineering for implantology: In Medicine and Dentistry. Walter de Gruyter GmbH & Co KG; 2022. [DOI:10.1515/9783110740134]
- Persaud A, Maus A, Strait L, Zhu D. 3D bioprinting with live cells. Engineered Regeneration. 2022; 3(3):292-309. [DOI:10.1016/j.engreg.2022.07.002]
- Hauptstein J, Böck T, Bartolf-Kopp M, Forster L, Stahlhut P, Nadernezhad A, et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Advanced Healthcare Materials. 2020; 9(15):2000737. [DOI:10.1002/adhm.202000737] [PMID]
- Abdollahiyan P, Oroojalian F, Mokhtarzadeh A, de la Guardia M. Hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Biotechnology Journal. 2020; 15(12):e2000095.[DOI:10.1002/biot.202000095] [PMID]
- Wang K, Wang Z, Hu H, Gao C. Supramolecular microgels/microgel scaffolds for tissue repair and regeneration. Supramolecular Materials. 2022; 1:100006. [DOI:10.1016/j.supmat.2021.100006]
- Piras CC, Fernández-Prieto S, De Borggraeve WM. Nanocellulosic materials as bioinks for 3D bioprinting. Biomaterials Science. 2017; 5(10):1988-92. [DOI:10.1039/C7BM00510E] [PMID]
- Li D, Zhou J, Zhang M, Ma Y, Yang Y, Han X, et al. Long-term delivery of alendronate through an injectable tetra-PEG hydrogel to promote osteoporosis therapy. Biomaterials Science. 2020; 8(11):3138-46. [DOI:10.1039/D0BM00376J] [PMID]
- Yu T, Wang H, Zhang Y, Wang X, Han B. The delivery of RNA-interference therapies based on engineered hydrogels for bone tissue regeneration. Frontiers in Bioengineering and Biotechnology. 2020; 8:445. [DOI:10.3389/fbioe.2020.00445] [PMID]
- Nedunchezian S, Banerjee P, Lee CY, Lee SS, Lin CW, Wu CW, et al. Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis. Materials Science and Engineering: C. 2021; 124:112072. [DOI:10.1016/j.msec.2021.112072] [PMID]
- Lam T, Dehne T, Krüger JP, Hondke S, Endres M, Thomas A, et al. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2019; 107(8):2649-57. [DOI:10.1002/jbm.b.34354] [PMID]
- Luo C, Xie R, Zhang J, Liu Y, Li Z, Zhang Y, et al. Low-temperature three-dimensional printing of tissue cartilage engineered with gelatin methacrylamide. Tissue Engineering Part C: Methods. 2020; 26(6):306-16. [DOI:10.1089/ten.tec.2020.0053] [PMID]
- Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Materials Science & Engineering. C, Materials for Biological Applications. 2018; 83:195-201. [DOI:10.1016/j.msec.2017.09.002] [PMID]
- Jammalamadaka U, Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. Journal of Functional Biomaterials. 2018; 9(1):22. [DOI:10.3390/jfb9010022] [PMID]
- Murphy C, Kolan K, Li W, Semon J, Day D, Leu M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. International Journal of Bioprinting. 2017; 3(1):005. [DOI:10.18063/IJB.2017.01.005] [PMID]
- Fakhraei O, Alimohammadi M, Moradi A, Akbarinezhad Nogh A, Soudmand Salarabadi S, Ghasabzadeh MS, et al. Nanofibrous polycaprolactone/chitosan membranes for preventing postsurgical tendon adhesion. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2022; 110(6):1279-91. [DOI:10.1002/jbm.b.34999] [PMID]
- Alimohammadi M, Fakhraei O, Moradi A, Kabiri M, Passandideh-Fard M, Tamayol A, et al. Controlled release of azithromycin from polycaprolactone/chitosan nanofibrous membranes. Journal of Drug Delivery Science and Technology. 2022; 71:103246. [DOI:10.1016/j.jddst.2022.103246]
- Mohan T, Maver T, Štiglic AD, Stana-Kleinschek K, Kargl R. 3D bioprinting of polysaccharides and their derivatives: From characterization to application. In: Thomas S, Balakrishnan P, Sreekala MS, editors. Fundamental biomaterials: polymers. A volume in Woodhead Publishing Series in Biomaterials. London: WP; 2018. [DOI:10.1016/B978-0-08-102194-1.00006-2]
- Wei P, Xu Y, Gu Y, Yao Q, Li J, Wang L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Delivery. 2020; 27(1):1106-14. [DOI:10.1080/10717544.2020.1797239] [PMID]
- Morris VB, Nimbalkar S, Younesi M, McClellan P, Akkus O. Mechanical properties, cytocompatibility and manufacturability of chitosan: PEGDA hybrid-gel scaffolds by stereolithography. Annals of Biomedical Engineering. 2017; 45(1):286-96.[DOI:10.1007/s10439-016-1643-1] [PMID]
- Kim JS, Hong S, Hwang C. Bio-ink materials for 3D bio-printing. Journal of International Society for Simulation Surgery. 2016; 3(2):49-59. [DOI:10.18204/JISSiS.2016.3.2.049]
- Wang H, Xu S, Fan D, Geng X, Zhi G, Wu D, et al. Multifunctional microcapsules: A theranostic agent for US/MR/PAT multi-modality imaging and synergistic chemo-photothermal osteosarcoma therapy. Bioactive Materials. 2022; 7:453-65. [DOI:10.1016/j.bioactmat.2021.05.004] [PMID]
- Bee SL, Hamid ZA, Mariatti M, Yahaya B, Lim K, Bee ST, et al. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: A review. Polymer Reviews. 2018; 58(3):495-536. [DOI:10.1080/15583724.2018.1437547]
- Li X, Liang Y, Xu X, Xiong J, Ouyang K, Duan L, et al. Cell-to-cell culture inhibits dedifferentiation of chondrocytes and induces differentiation of human umbilical cord-derived mesenchymal stem cells. BioMed Research International. 2019; 2019:5871698. [DOI:10.1155/2019/5871698] [PMID]
- Colle J, Blondeel P, De Bruyne A, Bochar S, Tytgat L, Vercruysse C, et al. Bioprinting predifferentiated adipose-derived mesenchymal stem cell spheroids with methacrylated gelatin ink for adipose tissue engineering. Journal of Materials Science: Materials in Medicine. 2020; 31(4):36. [DOI:10.1007/s10856-020-06374-w] [PMID]
- Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, Fukunishi T, et al. 3D bioprinting using stem cells. Pediatric Research. 2018; 83(1-2):223-31. [DOI:10.1038/pr.2017.252] [PMID]
- Ude CC, Miskon A, Idrus RBH, Abu Bakar MB. Application of stem cells in tissue engineering for defense medicine. Military Medical Research. 2018; 5(1):7. [DOI:10.1186/s40779-018-0154-9] [PMID]
- Grounds MD. Obstacles and challenges for tissue engineering and regenerative medicine: Australian nuances. Clinical and Experimental Pharmacology and Physiology. 2018; 45(4):390-400. [DOI:10.1111/1440-1681.12899] [PMID]
- Wragg NM, Burke L, Wilson SL. A critical review of current progress in 3D kidney biomanufacturing: Advances, challenges, and recommendations. Renal Replacement Therapy. 2019; 5:18. [DOI:10.1186/s41100-019-0218-7]
- Setayeshmehr M, Esfandiari E, Hashemibeni B, Tavakoli AH, Rafienia M, Samadikuchaksaraei A, et al. Chondrogenesis of human adipose-derived mesenchymal stromal cells on the [devitalized costal cartilage matrix/poly (vinyl alcohol)/fibrin] hybrid scaffolds. European Polymer Journal. 2019; 118:528-41. [DOI:10.1016/j.eurpolymj.2019.04.044]
- Beketov EE, Isaeva EV, Yakovleva ND, Demyashkin GA, Arguchinskaya NV, Kisel AA, et al. Bioprinting of cartilage with bioink based on high-concentration collagen and chondrocytes. International Journal of Molecular Sciences. 2021; 22(21):11351. [DOI:10.3390/ijms222111351] [PMID]
- Caddeo S, Boffito M, Sartori S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Frontiers in Bioengineering and Biotechnology. 2017; 5:40. [DOI:10.3389/fbioe.2017.00040] [PMID]
- Qu M, Jiang X, Zhou X, Wang C, Wu Q, Ren L, et al. Stimuli-responsive delivery of growth factors for tissue engineering. Advanced Healthcare Materials. 2020; 9(7):e1901714. [DOI:10.1002/adhm.201901714] [PMID]
- Mei Q, Rao J, Bei HP, Liu Y, Zhao X. 3D bioprinting photo-crosslinkable hydrogels for bone and cartilage repair. International Journal of Bioprinting. 2021; 7(3):367. [DOI:10.18063/ijb.v7i3.367] [PMID]
- Jain P, Kathuria H, Dubey N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials. 2022; 287:121639. [DOI:10.1016/j.biomaterials.2022.121639] [PMID]
- Augustine R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Progress in Biomaterials. 2018; 7(2):77-92. [DOI:10.1007/s40204-018-0087-0] [PMID]
- Rosser J, Thomas DJ. Bioreactor processes for maturation of 3D bioprinted tissue. In: Thomas DJ, Jessop ZM, Whitaker IS, editors. 3D Bioprinting for reconstructive surgery: Techniques and applications. London: WP; 2018. [DOI:10.1016/B978-0-08-101103-4.00010-7]
- König I, Soranno A, Nettels D, Schuler B. Impact of in-cell and in-vitro crowding on the conformations and dynamics of an intrinsically disordered protein. Angewandte Chemie. 2021; 133(19):10819-24. [DOI:10.1002/ange.202016804]
- Mousavi Nejad Z, Torabinejad B, Davachi SM, Zamanian A, Saeedi Garakani S, Najafi F, et al. Synthesis, physicochemical, rheological and in-vitro characterization of double-crosslinked hyaluronic acid hydrogels containing dexamethasone and PLGA/dexamethasone nanoparticles as hybrid systems for specific medical applications. International Journal of Biological Macromolecules. 2019; 126:193-208. [DOI:10.1016/j.ijbiomac.2018.12.181] [PMID]
- Cao Y, Cheng P, Sang S, Xiang C, An Y, Wei X, et al. Mesenchymal stem cells loaded on 3D-printed gradient poly (ε-caprolactone)/methacrylated alginate composite scaffolds for cartilage tissue engineering. Regenerative Biomaterials. 2021; 8(3):rbab019. [DOI:10.1093/rb/rbab019] [PMID]
- Rathan S, Dejob L, Schipani R, Haffner B, Möbius ME, Kelly DJ. Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage tissue engineering. Advanced Healthcare Materials. 2019; 8(7):1801501. [DOI:10.1002/adhm.201801501] [PMID]
- Zhang J, Hu Q, Wang S, Tao J, Gou M. Digital light processing based three-dimensional printing for medical applications. International Journal of Bioprinting. 2019; 6(1):242. [DOI:10.18063/ijb.v6i1.242] [PMID]
- Kosik-Kozioł A, Costantini M, Bolek T, Szöke K, Barbetta A, Brinchmann J, et al. PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration. Biofabrication. 2017; 9(4):044105. [DOI:10.1088/1758-5090/aa90d7] [PMID]
- Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, et al. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS One. 2017; 12(12):e0189428. [DOI:10.1371/journal.pone.0189428] [PMID]
- An S, Choi S, Min S, Cho SW. Hyaluronic acid-based biomimetic hydrogels for tissue engineering and medical applications. Biotechnology and Bioprocess Engineering. 2021; 26(4):503-16. [DOI:10.1007/s12257-020-0343-8]
- McMillan A, McMillan N, Gupta N, Kanotra SP, Salem AK. 3D bioprinting in otolaryngology: A review. Advanced Healthcare Materials. 2023; 12(19):e2203268. [DOI:10.1002/adhm.202203268] [PMID]
- Zopf DA, Mitsak AG, Flanagan CL, Wheeler M, Green GE, Hollister SJ. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngology--Head and Neck Surgery. 2015; 152(1):57-62. [DOI:10.1177/0194599814552065] [PMID]
- Skopinska-Wisniewska J, Tuszynska M, Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 2021; 14(2):396. [DOI:10.3390/ma14020396] [PMID]
- Huang J, Huang Z, Liang Y, Yuan W, Bian L, Duan L, et al. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomaterials Science. 2021; 9(7):2620-30. [DOI:10.1039/D0BM02103B] [PMID]
- Mushtaq F, Raza ZA, Batool SR, Zahid M, Onder OC, Rafique A, et al. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. International Journal of Biological Macromolecules. 2022; 218:601-33. [DOI:10.1016/j.ijbiomac.2022.07.168] [PMID]
- Rajabi N, Rezaei A, Kharaziha M, Bakhsheshi-Rad HR, Luo H, RamaKrishna S, et al. Recent advances on bioprinted gelatin methacrylate-based hydrogels for tissue repair. Tissue Engineering Part A. 2021; 27(11-12):679-702. [DOI:10.1089/ten.tea.2020.0350] [PMID]
- Gao Q, He Y, Fu JZ, Liu A, Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015; 61:203-15. [DOI:10.1016/j.biomaterials.2015.05.031] [PMID]
- Camacho P, Busari H, Seims KB, Tolbert JW, Chow LW. Materials as bioinks and bioink design. In: Guvendiren M, editor. 3D bioprinting in medicine. Cham: Springer; 2019. [DOI:10.1007/978-3-030-23906-0_2]
- Khalilifar MA, Eslaminejad MB, Ghasemzadeh M, Hosseini S, Baharvand H. In vitro and in vivo comparison of different types of rabbit mesenchymal stem cells for cartilage repair. Cell Journal. 2019; 21(2):150-60. [PMID]
- Zainal S, Mohd NH, Suhaili N, Anuar FH, Lazim AM, Othaman R. Preparation of cellulose-based hydrogel: A review. Journal of Materials Research and Technology. 2021; 10:935-52. [DOI:10.1016/j.jmrt.2020.12.012]
- Yin N, Chen SY, Cao YM, Wang HP, Wu QK. Improvement in mechanical properties and biocompatibility of biosynthetic bacterial cellulose/lotus root starch composites. Chinese Journal of Polymer Science. 2017; 35(3):354-64. [DOI:10.1007/s10118-017-1903-z]
- Pan T, Song W, Cao X, Wang Y. 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: Influence of crosslinking degree and pore architecture on physicochemical properties. Journal of Materials Science & Technology. 2016; 32(9):889-900. [DOI:10.1016/j.jmst.2016.01.007]
- Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Engineering Part B: Reviews. 2011; 17(4):281-99. [DOI:10.1089/ten.teb.2011.0077] [PMID]
- Shariatinia Z, Jalali AM. Chitosan-based hydrogels: Preparation, properties and applications. International Journal of Biological Macromolecules. 2018; 115:194-220. [DOI:10.1016/j.ijbiomac.2018.04.034] [PMID]
- Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. The Journal of Bone & Joint Surgery British Volume. 2008; 90(5):597-604. [DOI:10.1302/0301-620X.90B5.20360] [PMID]
- Do NH, Truong QT, Le PK, Ha AC. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydrate Polymers. 2022; 294:119726. [DOI:10.1016/j.carbpol.2022.119726] [PMID]
- Alimohammadi M, Aghli Y, Fakhraei O, Moradi A, Passandideh-Fard M, Ebrahimzadeh MH, et al. Electrospun nanofibrous membranes for preventing tendon adhesion. ACS Biomaterials Science & Engineering. 2020; 6(8):4356-76. [DOI:10.1021/acsbiomaterials.0c00201] [PMID]
- Chakraborty J, Mu X, Pramanick A, Kaplan DL, Ghosh S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials. 2022; 287:121672. [DOI:10.1016/j.biomaterials.2022.121672] [PMID]
- Taghizadeh M, Taghizadeh A, Yazdi M, Zarrintaj P, Stadler FJ, Ramsey JD, et al. Chitosan-based inks for 3D printing and bioprinting. Green Chemistry. 2022; 24(1):62-101. [DOI:10.1039/D1GC01799C]
- Tan XH, Liu L, Mitryashkin A, Wang Y, Goh JCH. Silk Fibroin as a Bioink-A thematic review of functionalization strategies for bioprinting applications. ACS Biomaterials Science & Engineering. 2022; 8(8):3242-70. [DOI:10.1021/acsbiomaterials.2c00313] [PMID]
- Gupta S, Alrabaiah H, Christophe M, Rahimi-Gorji M, Nadeem S, Bit A. Evaluation of silk-based bioink during pre and post 3D bioprinting: a review. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2021; 109(2):279-93. [DOI:10.1002/jbm.b.34699] [PMID]
- Ravanbakhsh H, Karamzadeh V, Bao G, Mongeau L, Juncker D, Zhang YS. Emerging technologies in multi-material bioprinting. Advanced Materials. 2021; 33(49):e2104730.[DOI:10.1002/adma.202104730] [PMID]
- Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomaterials Research. 2018; 22:11. [DOI:10.1186/s40824-018-0122-1] [PMID]
- Benders KE, Van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends in Biotechnology. 2013; 31(3):169-76. [DOI:10.1016/j.tibtech.2012.12.004] [PMID]
- Pountos I, Tellisi N, Ashammakhi N. Potential clinical applications of three-dimensional bioprinting. In: Guvendiren M, editor. 3D Bioprinting in Medicine: Technologies, Bioinks, and Applications. Berlin: Springer; 2019. [Link]
- Hauptstein J, Böck T, Bartolf-Kopp M, Forster L, Stahlhut P, Nadernezhad A, et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Advanced Healthcare Materials. 2020; 9(15):2000737. [PMID]
- Nedunchezian S, Banerjee P, Lee CY, Lee SS, Lin CW, Wu CW, et al. Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis. Materials Science & Engineering. C, Materials for Biological Applications. 2021; 124:112072. [PMID] [DOI:10.1016/j.msec.2021.112072]
- Mouser VH, Abbadessa A, Levato R, Hennink WE, Vermonden T, Gawlitta D, et al. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication. 2017; 9(1):015026. [DOI:10.1088/1758-5090/aa6265] [PMID]
- Bücking TM, Hill ER, Robertson JL, Maneas E, Plumb AA, Nikitichev DI. From medical imaging data to 3D printed anatomical models. Plos One. 2017; 12(5):e0178540. [DOI:10.1371/journal.pone.0178540] [PMID]
- Xu B, Yuan FZ, Lin L, Ye J, Fan BS, Zhang JY, et al. The higher inherent therapeutic potential of biomaterial-based hDPSCs and hEnSCs for pancreas diseases. Frontiers in Bioengineering and Biotechnology. 2020; 8:636. [PMID]
Review Paper: Review paper |
Subject:
General
Received: 2022/12/22 | Accepted: 2023/10/30 | Published: 2024/07/1
Received: 2022/12/22 | Accepted: 2023/10/30 | Published: 2024/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









