Volume 34, Issue 3 (10-2025)
JGUMS 2025, 34(3): 322-333 |
Back to browse issues page
Research code: 23509
Ethics code: IR.IUMS.FMD.REC.1401.173
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aghili N, Sobouti B. Diagnostic Accuracy of Procalcitonin for Distinguishing Between Gram-negative and Gram-positive Sepsis in Pediatric Burn Patients. JGUMS 2025; 34 (3) :322-333
URL: http://journal.gums.ac.ir/article-1-2749-en.html
URL: http://journal.gums.ac.ir/article-1-2749-en.html
1- Department of Pediatric, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 4220 kb]
(187 Downloads)
| Abstract (HTML) (909 Views)
Full-Text: (285 Views)
Introduction
Burn injuries break down the skin integrity, providing an ideal medium for bacterial proliferation and significantly increasing the risk of sepsis, which is a leading cause of morbidity and mortality in burn patients. Differentiating gram-negative sepsis from gram-positive sepsis is crucial, as gram-negative infections are often associated with higher inflammatory responses, increased organ dysfunction, and worse clinical outcomes compared to gram-positive infections. Blood culture, the gold standard for diagnosing sepsis, is time-consuming and may delay the initiation of appropriate antibiotic therapy. Biomarkers, such as serum procalcitonin (PCT), have emerged as valuable agents for distinguishing bacterial infections, considering their rapid elevation in systemic bacterial infections and their ability to guide early therapeutic decisions. This study aims to evaluate the diagnostic accuracy of PCT in distinguishing between gram-negative and gram-positive sepsis in pediatric burn patients.
Methods
This cross-sectional study included 117 pediatric patients (aged <14 years) with second- or third- or fourth-degree burns of total body surface area (TBSA), admitted to Shahid Motahari Burns Hospital in Tehran, Iran, between April and October 2022, diagnosed with sepsis. The sepsis diagnosis was based on the American Burn Association criteria including clinical signs and confirmed bloodstream infections. Blood cultures were prepared to isolate and identify the causative pathogens. The serum PCT level was measured using the chemiluminescence assay and analyzed along with clinical and laboratory data, such as inflammatory markers and organ dysfunction indicators. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic performance of PCT, and an optimal cut-off value was determined.
Results
The mean age of children was 70.28±59.79 months, and 70.3% were male. The mean burned TBSA was 40.55±14.92%, with most injuries being of a thermal nature (85.6%). Second-degree burns were observed in 37.3% of cases, while third-degree burns accounted for 61% of children. Only one case (0.8%) had a fourth-degree burn.
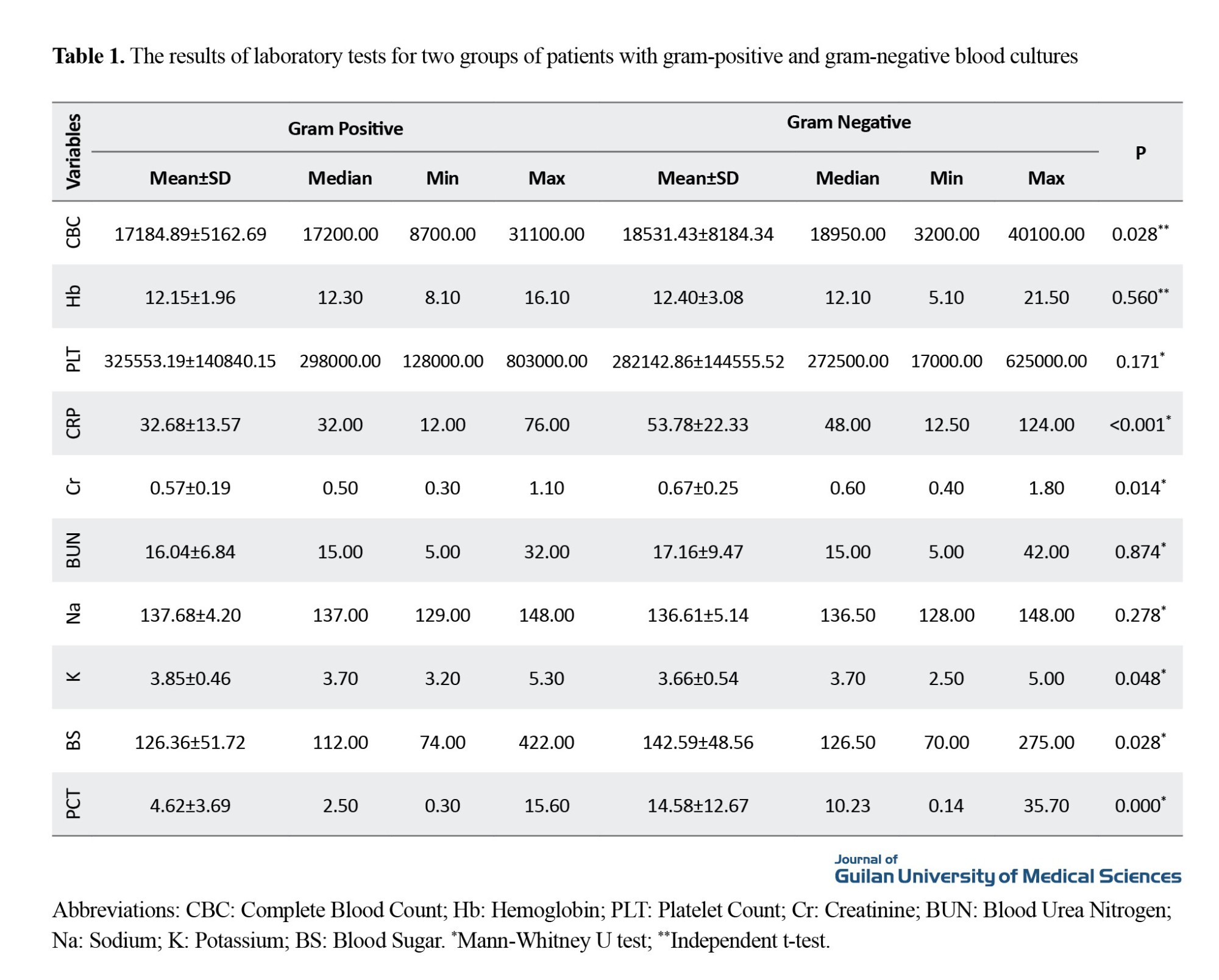
Gram-negative bacteria were isolated in 60.2% of patients, with Acinetobacter spp. (24.8%) and Pseudomonas spp. (23.1%) being the most common bacteria. In contrast, gram-positive bacteria were in 39.8% of patients, with Staphylococcus aureus (17.9%) and coagulase-negative staphylococci (22.2%) being the predominant isolates. The mean PCT level in patients with gram-negative sepsis was significantly higher compared to those with gram-positive sepsis (14.58±6.26 vs 4.62±2.67 ng/mL, P<0.001). Additionally, the C-reactive protein (CRP) levels were significantly elevated in the gram-negative group (53.78±17.41 vs 32.68±12.09 mg/L; P<0.001).
Other laboratory findings presented in Table 1, including elevated blood sugar (mean: 158.36 mg/dL in gram-negative vs 132.32 mg/dL in gram-positive; P=0.012), and increased serum creatinine level (0.67 mg/dL vs 0.57 mg/dL; P=0.014), indicated more severe organ dysfunction in the gram-negative group. The mean duration of hospitalization was also significantly longer in the gram-negative group (23.12±11.21 vs 18.42±10.69 days; P=0.026). The mortality rate in the gram-negative group was also significantly higher (31% vs 2%; P< 0.001).
The ROC curve analysis led to the determination of a PCT cut-off point of 3.15 ng/mL as optimal for differentiating gram-negative sepsis from gram-positive sepsis, with an area under the curve (AUC) of 0.762 (95% CI; 0.681%, 0.852%). At this threshold, the sensitivity and specificity were 84.3% and 57.4%, respectively. In a subgroup analysis based on burn severity, the AUC for PCT in patients with second-degree burns reached 0.904, with a cut-off point of 3.00 ng/mL, yielding a sensitivity of 88.9% and a specificity of 69.2%. These results underscore the clinical utility of PCT as a supportive diagnostic biomarker for gram-negative sepsis in pediatric burn patients, emphasizing the greater inflammatory burden, organ dysfunction, and mortality associated with gram-negative pathogens.
Conclusion
Serum PCT level is a valuable adjunct for distinguishing between gram-negative and gram-positive sepsis in pediatric burn patients, with moderate sensitivity and low specificity. Its diagnostic ability supports its role in guiding empirical antibiotic therapy and stratifying sepsis severity early at the time of hospitalization. However, PCT alone is insufficient for definitive diagnosis and should be combined with other clinical and laboratory indicators for optimal management. The findings emphasize the need for multicenter studies to validate the diagnostic thresholds and refine sepsis management protocols for burn patients at high risk of severe infections and adverse outcomes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1401.173).
Funding
This research did not receive any funding from funding organizations in the public, commercial, or nonprofit sectors.
Authors contributions
Conceptualization, validation, editing & review, visualization, supervision, and project administration: Behnam Sobouti; methodology, data analysis, investigation, and preparing the initial draft: Naeemeh Aghili and Behnam Sobouti.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the staff of Shahid Motahari Burns Hospital in Tehran for their support and assistance in data collection, and all participants for their cooperation in the study.
Burn injuries break down the skin integrity, providing an ideal medium for bacterial proliferation and significantly increasing the risk of sepsis, which is a leading cause of morbidity and mortality in burn patients. Differentiating gram-negative sepsis from gram-positive sepsis is crucial, as gram-negative infections are often associated with higher inflammatory responses, increased organ dysfunction, and worse clinical outcomes compared to gram-positive infections. Blood culture, the gold standard for diagnosing sepsis, is time-consuming and may delay the initiation of appropriate antibiotic therapy. Biomarkers, such as serum procalcitonin (PCT), have emerged as valuable agents for distinguishing bacterial infections, considering their rapid elevation in systemic bacterial infections and their ability to guide early therapeutic decisions. This study aims to evaluate the diagnostic accuracy of PCT in distinguishing between gram-negative and gram-positive sepsis in pediatric burn patients.
Methods
This cross-sectional study included 117 pediatric patients (aged <14 years) with second- or third- or fourth-degree burns of total body surface area (TBSA), admitted to Shahid Motahari Burns Hospital in Tehran, Iran, between April and October 2022, diagnosed with sepsis. The sepsis diagnosis was based on the American Burn Association criteria including clinical signs and confirmed bloodstream infections. Blood cultures were prepared to isolate and identify the causative pathogens. The serum PCT level was measured using the chemiluminescence assay and analyzed along with clinical and laboratory data, such as inflammatory markers and organ dysfunction indicators. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic performance of PCT, and an optimal cut-off value was determined.
Results
The mean age of children was 70.28±59.79 months, and 70.3% were male. The mean burned TBSA was 40.55±14.92%, with most injuries being of a thermal nature (85.6%). Second-degree burns were observed in 37.3% of cases, while third-degree burns accounted for 61% of children. Only one case (0.8%) had a fourth-degree burn.
Gram-negative bacteria were isolated in 60.2% of patients, with Acinetobacter spp. (24.8%) and Pseudomonas spp. (23.1%) being the most common bacteria. In contrast, gram-positive bacteria were in 39.8% of patients, with Staphylococcus aureus (17.9%) and coagulase-negative staphylococci (22.2%) being the predominant isolates. The mean PCT level in patients with gram-negative sepsis was significantly higher compared to those with gram-positive sepsis (14.58±6.26 vs 4.62±2.67 ng/mL, P<0.001). Additionally, the C-reactive protein (CRP) levels were significantly elevated in the gram-negative group (53.78±17.41 vs 32.68±12.09 mg/L; P<0.001).
Other laboratory findings presented in Table 1, including elevated blood sugar (mean: 158.36 mg/dL in gram-negative vs 132.32 mg/dL in gram-positive; P=0.012), and increased serum creatinine level (0.67 mg/dL vs 0.57 mg/dL; P=0.014), indicated more severe organ dysfunction in the gram-negative group. The mean duration of hospitalization was also significantly longer in the gram-negative group (23.12±11.21 vs 18.42±10.69 days; P=0.026). The mortality rate in the gram-negative group was also significantly higher (31% vs 2%; P< 0.001).

The ROC curve analysis led to the determination of a PCT cut-off point of 3.15 ng/mL as optimal for differentiating gram-negative sepsis from gram-positive sepsis, with an area under the curve (AUC) of 0.762 (95% CI; 0.681%, 0.852%). At this threshold, the sensitivity and specificity were 84.3% and 57.4%, respectively. In a subgroup analysis based on burn severity, the AUC for PCT in patients with second-degree burns reached 0.904, with a cut-off point of 3.00 ng/mL, yielding a sensitivity of 88.9% and a specificity of 69.2%. These results underscore the clinical utility of PCT as a supportive diagnostic biomarker for gram-negative sepsis in pediatric burn patients, emphasizing the greater inflammatory burden, organ dysfunction, and mortality associated with gram-negative pathogens.
Conclusion
Serum PCT level is a valuable adjunct for distinguishing between gram-negative and gram-positive sepsis in pediatric burn patients, with moderate sensitivity and low specificity. Its diagnostic ability supports its role in guiding empirical antibiotic therapy and stratifying sepsis severity early at the time of hospitalization. However, PCT alone is insufficient for definitive diagnosis and should be combined with other clinical and laboratory indicators for optimal management. The findings emphasize the need for multicenter studies to validate the diagnostic thresholds and refine sepsis management protocols for burn patients at high risk of severe infections and adverse outcomes.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1401.173).
Funding
This research did not receive any funding from funding organizations in the public, commercial, or nonprofit sectors.
Authors contributions
Conceptualization, validation, editing & review, visualization, supervision, and project administration: Behnam Sobouti; methodology, data analysis, investigation, and preparing the initial draft: Naeemeh Aghili and Behnam Sobouti.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors would like to thank the staff of Shahid Motahari Burns Hospital in Tehran for their support and assistance in data collection, and all participants for their cooperation in the study.
References
- World Health Organization (WHO). A WHO plan for burn prevention and care [Internet]. Geneva: World Health Organization, 2008. [Link]
- Weber J, McManus A; Nursing Committee of the International Society for Burn Injuries. Infection control in burn patients. Burns. 2004; 30(8):A16-24. [DOI:10.1016/j.burns.2004.08.003] [PMID]
- Mann EA, Wood GL, Wade CE. Use of procalcitonin for the detection of sepsis in the critically ill burn patient: A systematic review of the literature. Burns. 2011; 37(4):549-58. [DOI:10.1016/j.burns.2010.04.013] [PMID]
- Stanojcic M, Vinaik R, Jeschke MG. Status and challenges of predicting and diagnosing sepsis in burn patients. Surgical Infections. 2018; 19(2):168-75. [DOI:10.1089/sur.2017.288] [PMID]
- Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, et al. The leading causes of death after burn injury in a single pediatric burn center. Critical Care. 2009; 13(6):R183. [DOI:10.1186/cc8170] [PMID] [PMCID]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315(8):801-10. [DOI:10.1001/jama.2016.0287] [PMID] [PMCID]
- Horn DL, Morrison DC, Opal SM, Silverstein R, Visvanathan K, Zabriskie JB. What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clinical Infectious Diseases. 2000; 31(4):851-8. [DOI:10.1086/318127] [PMID]
- Tang A, Shi Y, Dong Q, Wang S, Ge Y, Wang C, et al. Prognostic differences in sepsis caused by gram-negative bacteria and gram-positive bacteria: A systematic review and meta-analysis. Critical Care. 2023; 27(1):467. [DOI:10.1186/s13054-023-04750-w] [PMID] [PMCID]
- Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clinical Microbiology Reviews. 2010; 23(1):235-51. [DOI:10.1128/CMR.00043-09] [PMID] [PMCID]
- Cabral L, Afreixo V, Meireles R, Vaz M, Frade JG, Chaves C, et al. Evaluation of procalcitonin accuracy for the distinction between gram-negative and gram-positive bacterial sepsis in burn patients. Journal of Burn Care & Research. 2019; 40(1):112-9. [DOI:10.1093/jbcr/iry058] [PMID]
- Broyles MR. Impact of procalcitonin-guided antibiotic management on antibiotic exposure and outcomes: real-world evidence. Open Forum Infectious Diseases. 2017; 4(4):ofx213. [DOI:10.1093/ofid/ofx213] [PMID] [PMCID]
- Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Medicine. 2003; 29(4):579-83. [DOI:10.1007/s00134-003-1664-8] [PMID]
- Lee WS, Kang DW, Back JH, Kim HL, Chung JH, Shin BC. Cutoff value of serum procalcitonin as a diagnostic biomarker of infection in end-stage renal disease patients. The Korean Journal of Internal Medicine. 2015; 30(2):198-204. [DOI:10.3904/kjim.2015.30.2.198] [PMID] [PMCID]
- Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Critical Care. 2018; 22(1):191. [DOI:10.1186/s13054-018-2125-7] [PMID] [PMCID]
- Vincent JL, Van Nuffelen M, Lelubre C. Host response biomarkers in sepsis: The role of procalcitonin. Methods in Molecular Biology. 2015; 1237:213-24. [DOI:10.1007/978-1-4939-1776-1_16] [PMID]
- Yiğit E, Demir Yiğit Y. Diagnostic importance of serum C-reactive protein and procalcitonin in sepsis after burn. International Journal of Burns and Trauma. 2021; 11(5):391-6. [PMID]
- Lari AR, Alaghehbandan R, Akhlaghi L. Burn wound infections and antimicrobial resistance in Tehran, Iran: An increasing problem. Annals of Burns and Fire Disasters. 2005; 18(2):68. [PMID]
- Moftian N, Rezaei-Hachesu P, Arab-Zozani M, Samad-Soltani T, Esfandiari A, Tabib MS, et al. Prevalence of gram-negative bacteria and their antibiotic resistance in neonatal sepsis in Iran: A systematic review and meta-analysis. BMc Infectious Diseases. 2023; 23(1):534. [DOI:10.1186/s12879-023-08508-1] [PMID] [PMCID]
- Jeschke MG, Finnerty CC, Kulp GA, Kraft R, Herndon DN. Can we use C-reactive protein levels to predict severe infection or sepsis in severely burned patients? International Journal of Burns and Trauma. 2013; 3(3):137-43. [PMID]
- Garofalo AM, Lorente-Ros M, Goncalvez G, Carriedo D, Ballén-Barragán A, Villar-Fernández A, et al. Histopathological changes of organ dysfunction in sepsis. Intensive Care Medicine Experimental. 2019; 7(Suppl 1):45. [DOI:10.1186/s40635-019-0236-3] [PMID] [PMCID]
- Faix JD. Biomarkers of sepsis. Critical Reviews in Clinical Laboratory Sciences. 2013; 50(1):23-36. [DOI:10.3109/10408363.2013.764490] [PMID] [PMCID]
- Ellithy M, Mitwally H, Saad M, Mathias R, Shaukat A, Elzeer H, et al. Mortality incidence among critically ill burn patients infected with multidrug-resistant organisms: A retrospective cohort study. Scars, Burns & Healing. 2021; 7:20595131211015133. [DOI:10.1177/20595131211015133] [PMID] [PMCID]
- Guo SY, Zhou Y, Hu QF, Yao J, Wang H. Procalcitonin is a marker of gram-negative bacteremia in patients with sepsis. The American Journal of the Medical Sciences. 2015; 349(6):499-504. [DOI:10.1097/MAJ.0000000000000477] [PMID] [PMCID]
- Pavel B, Popescu MR, Skolozubova D, Flutur E, Voiculescu VM, et al. Early low level of procalcitonin is associated with a favorable outcome in a case of a surviving patient with 80% body surface area thermal burn. The American Journal of Case Reports. 2021; 22:e934052. [DOI:10.12659/AJCR.934052] [PMID] [PMCID]
- Li S, Rong H, Guo Q, Chen Y, Zhang G, Yang J. Serum procalcitonin levels distinguish gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. Journal of Research in Medical Sciences . 2016; 21:39. [DOI:10.4103/1735-1995.183996] [PMID] [PMCID]
- Niu D, Huang Q, Yang F, Tian W, Li C, Ding L, et al. Serum biomarkers to differentiate gram-negative, gram-positive and fungal infection in febrile patients. Journal of Medical Microbiology. 2021; 70(7):001360. [DOI:10.1099/jmm.0.001360] [PMID]
- Liu HH, Zhang MW, Guo JB, Li J, Su L. Procalcitonin and C-reactive protein in early diagnosis of sepsis caused by either Gram-negative or Gram-positive bacteria. Irish Journal of Medical Science. 2017; 186(1):207-12. [DOI:10.1007/s11845-016-1457-z] [PMID]
- Leli C, Ferranti M, Moretti A, Al Dhahab ZS, Cenci E, Mencacci A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Disease Markers. 2015; 2015:701480. [DOI:10.1155/2015/701480] [PMID] [PMCID]
- Bilgili B, Haliloğlu M, Aslan MS, Sayan İ, Kasapoğlu US, Cinel İ. Diagnostic accuracy of procalcitonin for differentiating bacteraemic gram-negative sepsis from gram-positive sepsis. Turkish Journal of Anaesthesiology and Reanimation. 2018; 46(1):38-43. [PMID]
- Marik PE, Stephenson E. The ability of Procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database. Journal of Critical Care. 2020; 60:135-9. [DOI:10.1016/j.jcrc.2020.07.026] [PMID]
- Bakhtiar A, Haider Kazmi SJ, Asghar MS, Khurshaidi MN, Mazhar S, Khan NA, et al. Accuracy of procalcitonin levels for diagnosis of culture-positive sepsis in critically Ill trauma patients: A retrospective analysis. Cureus. 2021; 13(1):e12988. [DOI:10.7759/cureus.12988] [PMID] [PMCID]
Review Paper: Research |
Subject:
Special
Received: 2024/12/7 | Accepted: 2025/02/4 | Published: 2025/10/2
Received: 2024/12/7 | Accepted: 2025/02/4 | Published: 2025/10/2
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









