Volume 34, Issue 4 (12-2025)
JGUMS 2025, 34(4): 438-453 |
Back to browse issues page
Research code: 0
Ethics code: IR.YAZD.REC.1402.058
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zamani F, Nematzadeh Soteh N, Heidari M M, Chamani R, Khatami M, Moradi A. The Effect of Tumstatin and Canstatin-Derived Angiogenesis Inhibitor Peptides on FAK Expression in an Animal Model of Breast Cancer. JGUMS 2025; 34 (4) :438-453
URL: http://journal.gums.ac.ir/article-1-2776-en.html
URL: http://journal.gums.ac.ir/article-1-2776-en.html
Fatemeh Zamani1 

 , Niloofar Nematzadeh Soteh1
, Niloofar Nematzadeh Soteh1 

 , Mohammad Mehdi Heidari1
, Mohammad Mehdi Heidari1 

 , Reyhane Chamani *1
, Reyhane Chamani *1 

 , Mehri Khatami1
, Mehri Khatami1 

 , Ali Moradi2
, Ali Moradi2 




 , Niloofar Nematzadeh Soteh1
, Niloofar Nematzadeh Soteh1 

 , Mohammad Mehdi Heidari1
, Mohammad Mehdi Heidari1 

 , Reyhane Chamani *1
, Reyhane Chamani *1 

 , Mehri Khatami1
, Mehri Khatami1 

 , Ali Moradi2
, Ali Moradi2 


1- Department of Biology, Faculty of Science, Yazd University, Yazd, Iran.
2- Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
2- Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
Full-Text [PDF 6014 kb]
(87 Downloads)
| Abstract (HTML) (780 Views)
Full-Text: (77 Views)
Introduction
Canstatin and Tumstatin are endogenous angiogenesis inhibitor proteins derived from the α2 and α3 chains of collagen IV, respectively. They significantly prevent angiogenesis and tumor growth in vitro and animal models [13]. Two overlapping peptides from Tumstatin, T3 (amino acids 69–88) and T7 (amino acids 74–98), are more potent than other fragments [14]. Since short peptides may have advantages over longer ones in certain aspects, such as more convenient synthesis, we recently compared the antiangiogenic and antitumor activities of a 9-amino acid peptide derived from Tumstatin (amino acids 78-86) with its corresponding sequence in Canstatin. The results showed that both peptides significantly inhibited proliferation, migration, and vascular tube formation in endothelial cells, as well as colon tumor growth in mice.
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that is overexpressed and activated in several solid cancers, promoting tumor progression and metastasis. FAK controls cell motility, invasion, survival, and self-renewal of cancer stem cells [18]. FAK messenger ribonucleic acid (mRNA) levels are increased in several tumor types and inversely correlated with overall patient survival. Given the importance of FAK in the growth of cancer cells and clarification of the antitumor mechanism of peptides derived from Tumastatin (peptide T) and Canstatin (peptide C), this study aimed to investigate the effect of these peptides on breast tumor growth in mice and the expression of FAK in tumor tissue. No study has investigated the effect of peptides derived from these proteins on FAK expression. The present study sheds light on another aspect of their mechanism of action.
Methods
Peptides were synthesized by Shine Gene Biotechnology Inc. (Shanghai, China) with the amino acid sequence +3HN-LYCNPGDVC-COO- for peptide C and +3HN-LFCNVNDVC-COO- for peptide T.
A suspension containing 5×105 4T1 cells (a metastatic breast cell line) was injected s.c. into the right flank of mice. When the tumor volume reached approximately 200 mm3, tumor-bearing mice were randomly divided into control and treatment groups (n=3) and received phosphate-buffered saline (PBS) and 5 mg/kg/day of peptides (i.p. injection), respectively, for twelve days. Tumor dimensions were measured, and tumor volumes were calculated using the Equation 1:
1. v= a2×b×0.52,
where a is the smallest and b is the largest tumor diameter. Data were represented as Mean±SE.
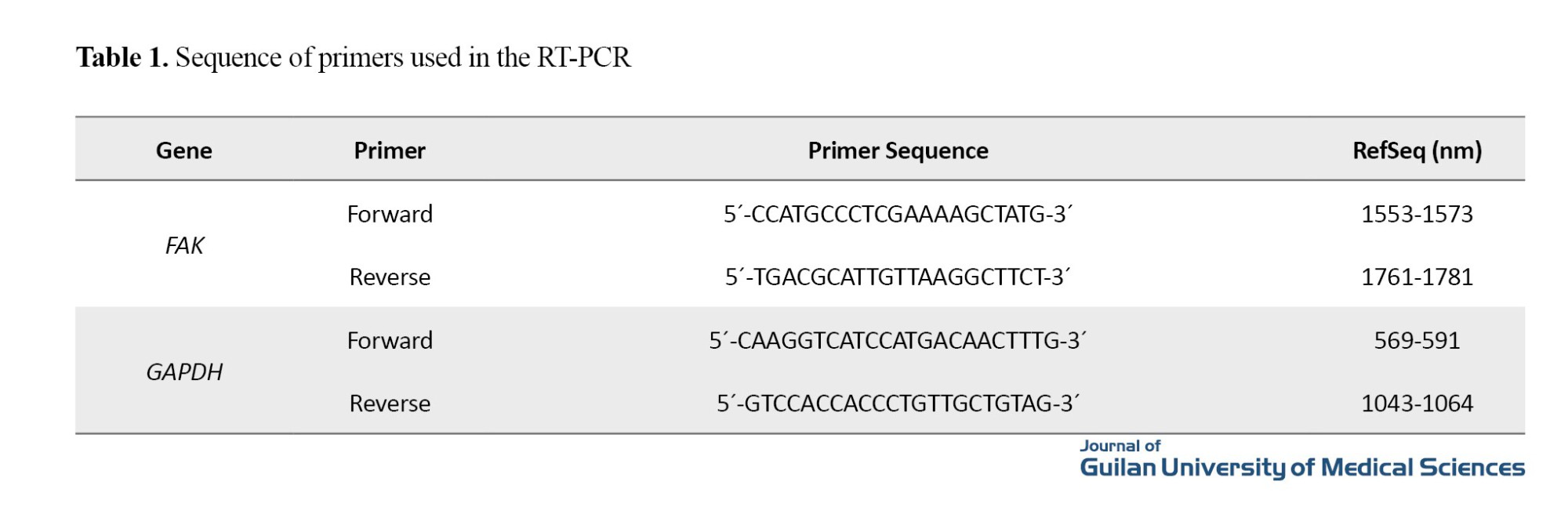
RNA was extracted from tumor tissues according to the manufacturer’s protocol. The primers required for the amplification of FAK and GAPDH genes (Table 1) were designed using this software. The Pars Toos kit was used for Complementary DNA (cDNA) synthesis. For real-time polymerase chain reaction (PCR), appropriate volumes of Mastermix, cDNA, and primers were mixed and brought up to 20 μL with sterile double-distilled water. The four-step PCR was programmed as follows: A 15-minute holding step at 95 °C, an initial 30-second denaturation step at 95 °C, a 30-second annealing step at 65 °C, and a 30-second extension step at 72 °C. All programs were run with at least three independent cDNA samples. Finally, the cycle threshold (CT) values of genes in the treated and control samples were calculated. The CT of the FAK was subtracted from the CT of the GAPDH, and the ΔCT value was obtained for the treated and control samples. The relative expression graph was displayed based on 2-ΔCT. Data were represented as Mean±SE. Statistical significance was set at P<0.05.
Results
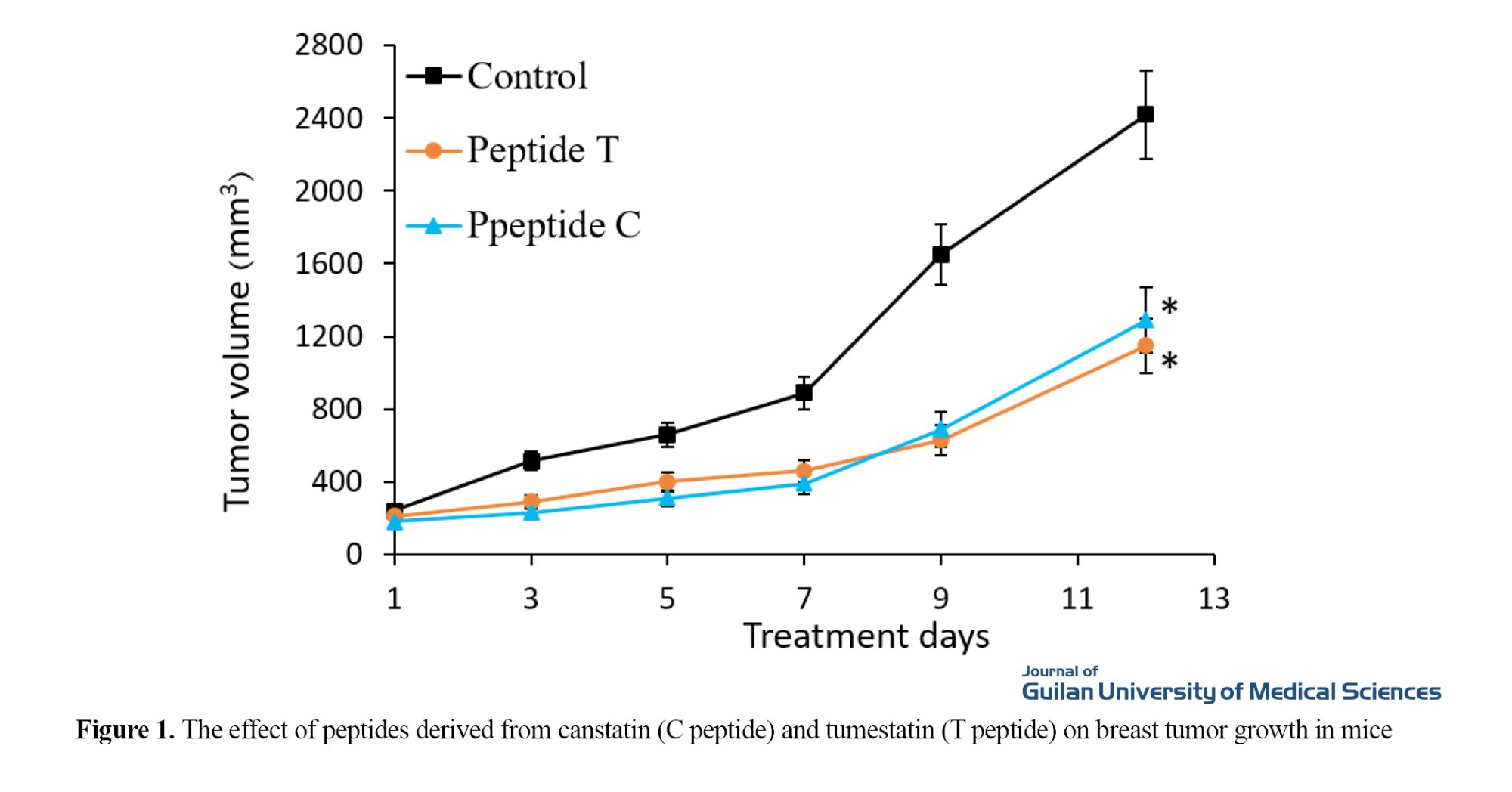
Peptides C and T prevented tumor growth by 51% and 49%, respectively, compared to the control group (Figure 1) (P<0.05).
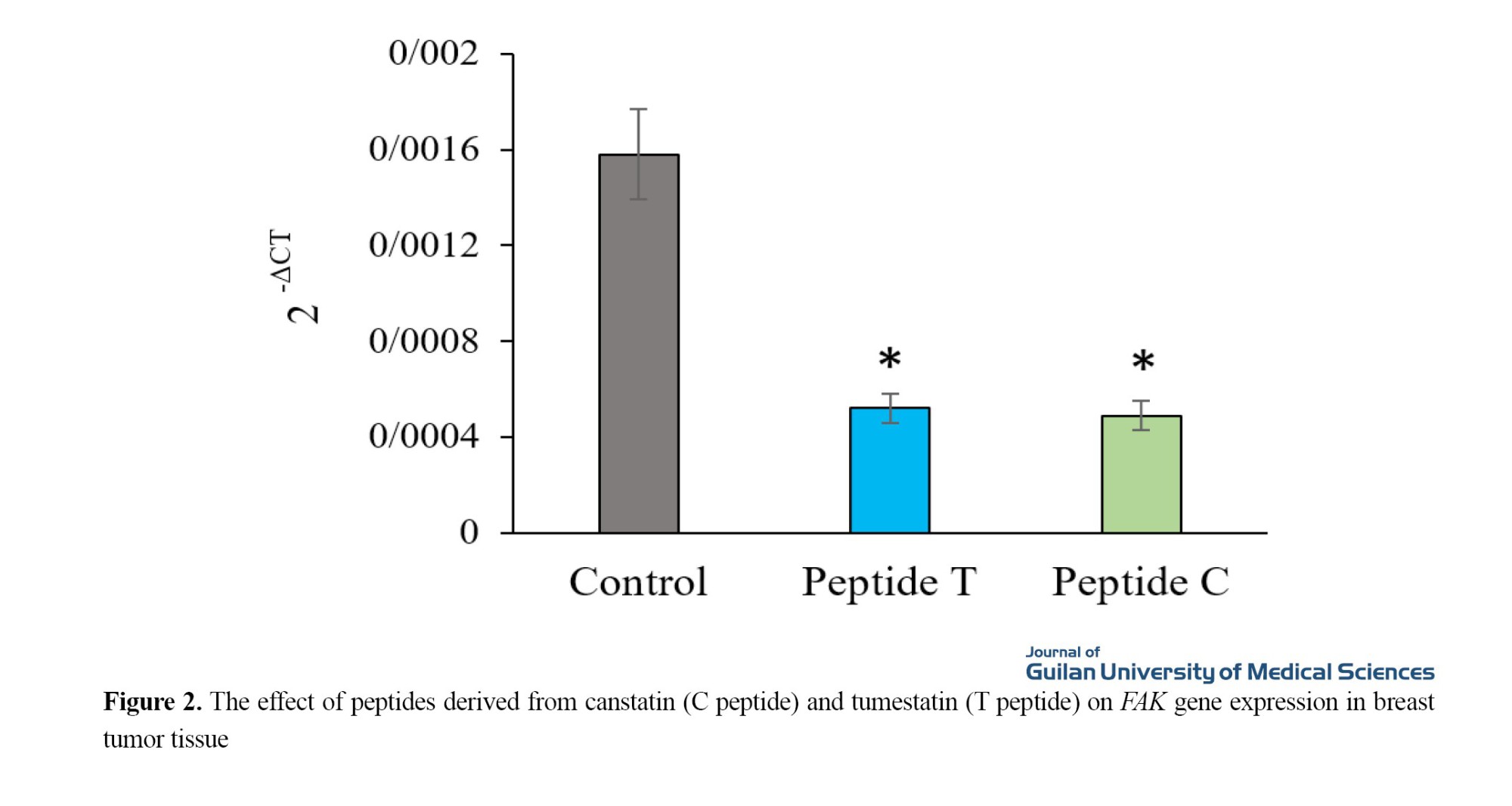
However, the difference between the two treatment groups was not significant. Peptides C and T reduced FAK expression by 69% and 67%, respectively, compared to the control group (P<0.05) (Figure 2). No significant difference was observed between the effects of the two peptides on FAK expression.
Conclusion
The present study showed that short antiangiogenic peptides derived from collagen IV can inhibit breast tumor growth in mice by approximately 50% and reduce the expression of FAK, a crucial gene involved in the growth, proliferation, and metastasis of cancer cells. Given the anticancer effects of these peptides, which also have potential applications in tumor diagnosis and targeted drug delivery, further preclinical studies to determine their optimal dosage and toxicity would be beneficial.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Yazd University, Yazd, Iran (Code: IR.YAZD.REC.1402.058).
Funding
This article was extracted from master's thesis of Fatemeh Zamani and Niloofar Nematzadeh, approved by Department of Biology, Yazd University, Yazd, Iran.
Authors contributions
Conceptualization, study design and study supervision: Reyhane Chamani and Mohammad Mehdi Heidari; Data collection, data analysis and Statistical analysis: Fatemeh Zamani and Niloofar Nematzadeh Soteh; Drafting of the manuscript: Reyhane Chamani and Mehri Khatami; Critical revision of the manuscript for important intellectual content: Mohammad Mehdi Heidari and Ali Moradi; Administrative, technical, or material support: Ali Moradi.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors are gratefully acknowledge the support of the Research and Technology Deputy of Yazd University for their assistance in conducting this study.
Canstatin and Tumstatin are endogenous angiogenesis inhibitor proteins derived from the α2 and α3 chains of collagen IV, respectively. They significantly prevent angiogenesis and tumor growth in vitro and animal models [13]. Two overlapping peptides from Tumstatin, T3 (amino acids 69–88) and T7 (amino acids 74–98), are more potent than other fragments [14]. Since short peptides may have advantages over longer ones in certain aspects, such as more convenient synthesis, we recently compared the antiangiogenic and antitumor activities of a 9-amino acid peptide derived from Tumstatin (amino acids 78-86) with its corresponding sequence in Canstatin. The results showed that both peptides significantly inhibited proliferation, migration, and vascular tube formation in endothelial cells, as well as colon tumor growth in mice.
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that is overexpressed and activated in several solid cancers, promoting tumor progression and metastasis. FAK controls cell motility, invasion, survival, and self-renewal of cancer stem cells [18]. FAK messenger ribonucleic acid (mRNA) levels are increased in several tumor types and inversely correlated with overall patient survival. Given the importance of FAK in the growth of cancer cells and clarification of the antitumor mechanism of peptides derived from Tumastatin (peptide T) and Canstatin (peptide C), this study aimed to investigate the effect of these peptides on breast tumor growth in mice and the expression of FAK in tumor tissue. No study has investigated the effect of peptides derived from these proteins on FAK expression. The present study sheds light on another aspect of their mechanism of action.
Methods
Peptides were synthesized by Shine Gene Biotechnology Inc. (Shanghai, China) with the amino acid sequence +3HN-LYCNPGDVC-COO- for peptide C and +3HN-LFCNVNDVC-COO- for peptide T.
A suspension containing 5×105 4T1 cells (a metastatic breast cell line) was injected s.c. into the right flank of mice. When the tumor volume reached approximately 200 mm3, tumor-bearing mice were randomly divided into control and treatment groups (n=3) and received phosphate-buffered saline (PBS) and 5 mg/kg/day of peptides (i.p. injection), respectively, for twelve days. Tumor dimensions were measured, and tumor volumes were calculated using the Equation 1:
1. v= a2×b×0.52,
where a is the smallest and b is the largest tumor diameter. Data were represented as Mean±SE.
RNA was extracted from tumor tissues according to the manufacturer’s protocol. The primers required for the amplification of FAK and GAPDH genes (Table 1) were designed using this software. The Pars Toos kit was used for Complementary DNA (cDNA) synthesis. For real-time polymerase chain reaction (PCR), appropriate volumes of Mastermix, cDNA, and primers were mixed and brought up to 20 μL with sterile double-distilled water. The four-step PCR was programmed as follows: A 15-minute holding step at 95 °C, an initial 30-second denaturation step at 95 °C, a 30-second annealing step at 65 °C, and a 30-second extension step at 72 °C. All programs were run with at least three independent cDNA samples. Finally, the cycle threshold (CT) values of genes in the treated and control samples were calculated. The CT of the FAK was subtracted from the CT of the GAPDH, and the ΔCT value was obtained for the treated and control samples. The relative expression graph was displayed based on 2-ΔCT. Data were represented as Mean±SE. Statistical significance was set at P<0.05.

Results
Peptides C and T prevented tumor growth by 51% and 49%, respectively, compared to the control group (Figure 1) (P<0.05).

However, the difference between the two treatment groups was not significant. Peptides C and T reduced FAK expression by 69% and 67%, respectively, compared to the control group (P<0.05) (Figure 2). No significant difference was observed between the effects of the two peptides on FAK expression.

Conclusion
The present study showed that short antiangiogenic peptides derived from collagen IV can inhibit breast tumor growth in mice by approximately 50% and reduce the expression of FAK, a crucial gene involved in the growth, proliferation, and metastasis of cancer cells. Given the anticancer effects of these peptides, which also have potential applications in tumor diagnosis and targeted drug delivery, further preclinical studies to determine their optimal dosage and toxicity would be beneficial.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Yazd University, Yazd, Iran (Code: IR.YAZD.REC.1402.058).
Funding
This article was extracted from master's thesis of Fatemeh Zamani and Niloofar Nematzadeh, approved by Department of Biology, Yazd University, Yazd, Iran.
Authors contributions
Conceptualization, study design and study supervision: Reyhane Chamani and Mohammad Mehdi Heidari; Data collection, data analysis and Statistical analysis: Fatemeh Zamani and Niloofar Nematzadeh Soteh; Drafting of the manuscript: Reyhane Chamani and Mehri Khatami; Critical revision of the manuscript for important intellectual content: Mohammad Mehdi Heidari and Ali Moradi; Administrative, technical, or material support: Ali Moradi.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors are gratefully acknowledge the support of the Research and Technology Deputy of Yazd University for their assistance in conducting this study.
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA. 2024; 74(1):12-49. [DOI:10.3322/caac.21820] [PMID]
- Hida K, Maishi N, Matsuda A, Yu L. Beyond starving cancer: Anti-angiogenic therapy. Journal of Medical Ultrasonics. 2024; 51(4):605-10. [DOI:10.1007/s10396-023-01310-1] [PMID]
- Ansari MJ, Bokov D, Markov A, Jalil AT, Shalaby MN, Suksatan W, et al. Cancer combination therapies by angiogenesis inhibitors; A comprehensive review. Cell Communication and Signaling. 2022; 20(1):49. [DOI:10.1186/s12964-022-00838-y] [PMID]
- Sarabi N, Chamani R, Assareh E, Saberi O, Asghari SM. Combination therapy in cancer: doxorubicin in combination with an n-terminal peptide of endostatin suppresses angiogenesis and stimulates apoptosis in the breast cancer. International Journal of Molecular and Cellular Medicine. 2023; 12(2):120. [DOI:10.22088/IJMCM.BUMS.12.2.120] [PMID]
- Tan X, Yan Y, Song B, Zhu S, Mei Q, Wu K. Focal adhesion kinase: From biological functions to therapeutic strategies. Experimental Hematology & Oncology. 2023; 12(1):83. [DOI:10.1186/s40164-023-00446-7] [PMID]
- Chuang HH, Zhen YY, Tsai YC, Chuang CH, Hsiao M, Huang MS, et al. FAK in Cancer: From mechanisms to therapeutic strategies. International Journal of Molecular Sciences. 2022; 23(3):1726. [DOI:10.3390/ijms23031726] [PMID]
- Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, et al. Therapeutic peptides: Current applications and future directions. Signal Transduction and Targeted Therapy. 2022; 7(1):48. [DOI:10.1038/s41392-022-00904-4] [PMID]
- Rehanandeh M, Salehi Z, Asghari SM. [In a caspase-and caspase-9 mouse model, the effect of antiangiogenic peptides on the expression of 3 4T genes with induced breast cancer with Balb/c cell line 1 (Persian)]. Journal of Animal Environment. 2019; 11(4):83-8. [Link]
- Kaboudan F, Talesh Sasani S, Asghari SM. [The effect of a VEGFB antagonist peptide on the expression level of miR-210 in a mouse model of breast cancer (Persian)]. Nova Biologica Reperta. 2021; 8(1):13-9. [DOI:10.52547/NBR.8.1.13]
- Sharma K, Sharma KK, Sharma A, Jain R. Peptide-based drug discovery: Current status and recent advances. Drug Discovery Today. 2023; 28(2):103464. [DOI:10.1016/j.drudis.2022.103464] [PMID]
- Bielajew BJ, Hu JC, Athanasiou KA. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nature Reviews. Materials. 2020; 5(10):730-47. [DOI:10.1038/s41578-020-0213-1] [PMID]
- Okada M, Yamawaki H. A current perspective of canstatin, a fragment of type IV collagen alpha 2 chain. Journal of Pharmacological Sciences. 2019; 139(2):59-64. [DOI:10.1016/j.jphs.2018.12.001] [PMID]
- Panka DJ, Mier JW. Canstatin inhibits Akt activation and induces Fas-dependent apoptosis in endothelial cells. The Journal of Biological Chemistry. 2003; 278(39):37632-6. [DOI:10.1074/jbc.M307339200] [PMID]
- Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Researchs. 2005; 65(10):4353-61. [DOI:10.1158/0008-5472.CAN-04-3536] [PMID]
- He GA, Luo JX, Zhang TY, Hu ZS, Wang FY. The C-terminal domain of canstatin suppresses in vivo tumor growth associated with proliferation of endothelial cells. Biochemical and Biophysical Research Communications. 2004; 318(2):354-60. [DOI:10.1016/j.bbrc.2004.04.038] [PMID]
- Sudhakar A, Boosani CS. Inhibition of tumor angiogenesis by tumstatin: Insights into signaling mechanisms and implications in cancer regression. Pharmaceutical Research. 2008; 25(12):2731-9. [DOI:10.1007/s11095-008-9634-z] [PMID]
- Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, et al. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. The Journal of Biological Chemistry. 2001; 276(34):31959-68. [DOI:10.1074/jbc.M103024200] [PMID]
- Eikesdal HP, Sugimoto H, Birrane G, Maeshima Y, Cooke VG, Kieran M, et al. Identification of amino acids essential for the antiangiogenic activity of tumstatin and its use in combination antitumor activity. Proceedings of the National Academy of Sciences of the United States of America. 2008; 105(39):15040-5. [DOI:10.1073/pnas.0807055105] [PMID]
- Grafton KT, Moir LM, Black JL, Hansbro NG, Hansbro PM, Burgess JK, et al. LF-15 & T7, synthetic peptides derived from tumstatin, attenuate aspects of airway remodelling in a murine model of chronic OVA-induced allergic airway disease. Plos One. 2014; 9(1):e85655. [DOI:10.1371/journal.pone.0085655] [PMID]
- Chamani R, Taleqani MH, Imanpour A, Khatami M. New insights into short peptides derived from the collagen NC1 α1, α2, and α3 (IV) domains: An experimental and MD simulations study. Biochimica et Biophysica Acta. Proteins and Proteomics. 2022; 1870(4):140769. [DOI:10.1016/J.BBAPAP.2022.140769] [PMID]
- Motamedi F, Khatami M, Heidari MM, Azadeh M, Karami N. Evaluation of the expression of LINCOO475 and FOXO1 genes as tumor suppressor genes in breast cancer: correlation of bioinformatics tools and experimental approaches. Gene Reports. 2024; 36:101980. [DOI:10.1016/J.GENREP.2024.101980]
- Mohammadpanah M, Heidari MM, Khatami M, Hadadzadeh M. Relationship of hypomethylation CpG islands in interleukin-6 gene promoter with IL-6 mRNA levels in patients with coronary atherosclerosis. Journal of Cardiovascular and Thoracic Research. 2020; 12(3):214-21. [DOI:10.34172/JCVTR.2020.37] [PMID]
- Kawaguchi T, Yamashita Y, Kanamori M, Endersby R, Bankiewicz KS, Baker SJ, et al. The PTEN/Akt pathway dictates the direct alphaVbeta3-dependent growth-inhibitory action of an active fragment of tumstatin in glioma cells in vitro and in vivo. Cancer Research. 2006; 66(23):11331-40. [DOI:10.1158/0008-5472.CAN-06-1540] [PMID]
- Thevenard J, Floquet N, Ramont L, Prost E, Nuzillard JM, Dauchez M, et al. Structural and antitumor properties of the YSNSG cyclopeptide derived from tumstatin. Chemistry & Biology. 2006; 13(12):1307-15. [DOI:10.1016/j.chembiol.2006.10.007] [PMID]
- He GA, Luo JX, Zhang TY, Wang FY, Li RF. Canstatin-N fragment inhibits in vitro endothelial cell proliferation and suppresses in vivo tumor growth. Biochemical and Biophysical Research Communications. 2003; 312(3):801-5. [DOI:10.1016/j.bbrc.2003.11.003] [PMID]
- Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, Popel AS. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Scientific Reports. 2014; 4:7139. [DOI:10.1038/srep07139] [PMID]
- Barbhuiya MA, Mirando AC, Simons BW, Lemtiri-Chlieh G, Green JJ, Popel AS, et al. Therapeutic potential of an anti-angiogenic multimodal biomimetic peptide in hepatocellular carcinoma. Oncotarget. 2017; 8(60):101520-34. [DOI:10.18632/oncotarget.21148] [PMID]
- Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, et al. Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer. 2010; 10:29. [DOI:10.1186/1471-2407-10-29] [PMID]
- Sadremomtaz A, Kobarfard F, Mansouri K, Mirzanejad L, Asghari SM. Suppression of migratory and metastatic pathways via blocking VEGFR1 and VEGFR2. Journal of Receptor and Signal Transduction Research. 2018; 38(5-6):432-41. [DOI:10.1080/10799893.2019.1567785] [PMID]
- Schlaepfer DD, Ojalill M, Stupack DG. Focal adhesion kinase signaling-tumor vulnerabilities and clinical opportunities. Journal of Cell Science. 2024; 137(14):jcs261723. [DOI:10.1242/jcs.261723] [PMID]
- Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nature Reviews. Cancer. 2020; 20(8):471-80. [DOI:10.1038/s41568-020-0262-1] [PMID]
- Chamani R, Saberi O, Fathinejad F. An arresten-derived anti-angiogenic peptide triggers apoptotic cell death in endothelial cells. Molecular Biology Reports. 2024; 51(1):513. [DOI:10.1007/s11033-024-09448-y] [PMID]
- Chamani R, Zamani F. Novel anti-angiogenic peptide derived from canstatin induces apoptosis in vitro and in vivo. International Journal of Peptide Research and Therapeutics. 2022; 28(5):149. [DOI:10.1007/s10989-022-10458-2]
- Gori A, Lodigiani G, Colombarolli SG, Bergamaschi G, Vitali A. Cell Penetrating Peptides: Classification, mechanisms, methods of study, and applications. ChemMedChem. 2023; 18(17):e202300236. [DOI:10.1002/cmdc.202300236] [PMID]
Review Paper: Research |
Subject:
Special
Received: 2025/03/1 | Accepted: 2025/05/5 | Published: 2026/01/1
Received: 2025/03/1 | Accepted: 2025/05/5 | Published: 2026/01/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






